Editorial Review
May 2019, 26:2
First online: 27 May 2019
Editorial Review
Cardiovascular polypharmacy for atherosclerotic cardiovascular disease, therapeutic goals and targets: Are we doing the right thing?
David KL Quek,1 FRCP, FNHAM, FAsCC, FESC, FACC
“Healthcare is dominated by long term conditions, there is little curing any more. Medicine is about ameliorating, palliating, listening, explaining, advising, and consoling. It’s not glamorous. It should also be about caring, but patients accept that doctors are “too busy” for that (sometimes, I fear, in pursuit of the mirage of diagnosing, treating and curing)… But now most of us are incurable…” ~ Richard Smith, former Editor-in-chief, BMJ1
I recently presented a lecture using a case illustration of a cardiac patient presenting with recurrent myocardial ischemia. He is diabetic with multiple cardiovascular (CV) risk factors. This case discussion was to an international group of internists, physicians and endocrinologists, attending the International Diabetic Federation Conference 2018, in Kuala Lumpur.
My impression from the number of comments and queries that arose during the interactive lecture was that many of these physicians were alarmed as to the number of medications that this patient had been prescribed, no matter the complex cardiovascular comorbidities that this patient had. The caution from this group of mainly diabetic specialists was that perhaps there was excessive ‘polypharmacy’ involved in the management of this patient.
As physicians, we could not agree more. But I would argue that as physicians we should practice as per guidelines for the overall long term good of our patients. We must learn to treat to established targets and goals that have been shown to not only improve and reduce microvascular but macrovascular complications, but also to attain better quality of life, and longer survival, free from major adverse CV events, i.e. MACE. Being in the front line where complications and sequelae of major adverse CV events present, cardiologists are best placed to recognise the urgencies and indeed the need for more aggressive management of the multi-morbid patients.
Lest it is forgotten, CV deaths including sudden cardiac death, heart failure from chronic myocardial ischemia or acute coronary syndromes still affect most diabetic patients. The recent TECOS study showed that some 49% of a contemporary cohort of diabetic patients with ASCVD die of some CV causes, i.e. sudden cardiac death, acute myocardial infarction/stroke and heart failure.2
It is no longer acceptable to treat each diagnostic group or comorbidity in silos, without the all-inclusive view for long-term prognosis. We are treating the patient, and not just the narrow disease entity. Thus, my contention is that it is not reckless polypharmacy per se, because we are trying to apply the best science and evidence for the patient. We would like to see our patients live well and long into the future by ameliorating the ‘natural’ history of adverse consequences/ sequelae from his chronic ailments.
EMPHASIZE NON-PHARMACOLOGICAL LIFESTYLE INTERVENTION
Every iteration of disparate clinical practice guideline constantly reminds us physicians that there must be more counselling and lifestyle intervention to modify and improve our patient’s health outcomes. I acknowledge that I did not emphasize in the talk about routine counselling for risk modification of lifestyle. However, lifestyle intervention practice is de rigueur and sine qua non, as part of nonpharmacological therapy. It is inalienable part of our normal duties and responsibilities as physicians. Having said that, such behavioural modification is difficult to achieve in a non-motivated patient; indeed, for most of our patients.
The beneficial results of behavioural intervention are clear in welldefined interactive programmes.3 Interventions that were studied included increased physical activity, reduced caloric intake, dietary education, and counselling and education regarding treatment adherence or disease monitoring. There were many inconsistencies, but overall there were significant if modest improvement with some CV risk factors such as BMI, HbA1c, systolic and diastolic blood pressure. LDL-C and HDL-C levels were not significantly changed by lifestyle intervention per se.3
However, most lifestyle interventions have to be well-structured and intensive, with dietary counselling and exercise programmes being the emphases for intervention.4 Discrepancies about what works and what does not, exist and are being debated. Brand T et al,5 found that self-monitoring, group-based components, and motivational signs to encourage stair use, were identified as promising strategies to increase physical activity, especially with children and adolescents. Among adults at risk for type II diabetes, evidence for beneficial effects was limited to weight change and diabetes incidence. However, evidence for effective interventions aimed at general adult populations were by and large inconclusive. Hence, community-based interventions should start from young age i.e. school-going children, to instil a lifetime habit of healthy eating and more physical activity.
However, the persistence or adherence of lifestyle intervention/ modification in the longer term, has not been well studied or documented.[6] Notwithstanding this, can episodic or short term CV risk factor intervention be translated into longer prognosis of having fewer adverse cardiovascular outcomes? This remains unclear and untested.
In day-to-day clinical practice, failure to adhere to long term behavioural intervention, is the norm rather than otherwise. Recidivism in terms of poor dietary habits, smoking and physical inactivity are almost the rule. But there was disquieting silence when it came to prognostic implications of these more conservative arguments, particularly the difficulty in finding high quality evidence to back up these behavioural modifications in a patient with multiple comorbidities, and those obviously having very high cardiovascular risk.
I had presented this case to highlight the complexities of managing a multiply-afflicted patient, with a not uncommon cluster of chronic ailments that now needs better assessment and management. I discussed the growing lists of chronic disease guidelines that urge physicians to be more mindful and thorough, so that we can hope to delay and reduce major adverse cardiovascular events and deaths. This is still our primary remit as physicians.
Most if not all major Guidelines indeed advocate that we should manage patients as holistically as possible. We must consider all the CV risk factors and co-morbidities, treat them well, if we hope to arrest, defer or even to reverse, the relentless disease progression in very high-risk patients who would otherwise have very poor prognoses.
I am sharing this case illustration to invite comments and perhaps raise more than a few questions, as to how we can best manage a growing disease burden, i.e. chronic ischaemic heart disease. We are experiencing more noncommunicable chronic disease (NCVD) in this modern era, where there are ever more patients with multiple comorbidities.
Indeed, how we can be more prudent or circumspect in our therapies? Are we overtreating, overmedicalizing our patients? Indeed, are we making our patients’ lives better, or just more miserable, having to consume so many pills just to adhere to our guideline-driven ethos?
CASE ILLUSTRATION
SDR, was a 50-year old man who first presented in 2007 for recurrent and progressive angina. He had been an inveterate smoker (25 cigarettes/day) for 35+ years, known hypertensive 7 years (on and off treatment), early impaired fasting glucose (Fasting glucose 7.4 mol/l, HbA1c 6.8%). His father died at 50 years old purportedly from a road traffic accident, and his mother died of chronic diabetic kidney disease in her 40s. His blood lipid profile was as follows: total cholesterol (TC) 6.2 mmol/l, triglycerides (TG) 2.8 mmol/l, HDL-C 0.9 mmol/l, LDL-C 4.0 mmol/l, non-HDL-C 5.3 mmol/l.
He had a strongly positive treadmill stress test, that led to coronary angiography in January 2008. He was found to have a near total occlusion (99%) at mid to distal RCA, as well as a mid-Circumflex artery stenosis of 90%. There was a non-critical 30% plaque in the mid LAD. Stent-angioplasty was performed to revascularize the RCA and the Circumflex with optimum results. His medications at discharge were aspirin 300 mg daily, clopidogrel 75 mg daily, bisoprolol 5 mg daily, valsartan hydrochlorothiazide 160/12.5 mg daily, metformin SR 750 mg daily, atorvastatin 80 mg daily.
He was reviewed twice in 2008, once in 2009, and was found stable. But after 2010, he was lost to follow up. He re-presented with recurrent effort angina in January 2016. What worried him was that his younger brother of 53 years then, had just had a heart attack and had primary angioplasty; he also had diabetes.
SDR (now 59 years old) was still smoking 20 cigarettes/day (he had tried hard to quit, but failed again and again), he had gained weight 78kg for his height of 170cm, his BMI was now 27 and his waist circumference was 89cm. His BP was 155/94 mmHg, heart rate was 68 beats/min, regular, his peripheral pulses were normal.
There were no other abnormal physical signs. He had let his community pharmacist modified his medications to a fixed-dose combination of glibenclamide 2.5 mg + metformin 500 mg BID for his diabetes, amlodipine 5 mg OD, metoprolol 25 mg OD, for his hypertension, Aspirin 100 mg OD, and lovastatin 10 mg OD for his CVD prophylaxis. His current blood workup revealed that his lipid profile was suboptimal: TC 5.4 mmol/l, TG 2.7 mmol/l, HDL-C 1.0 mmol/l, LDL-C 3.2 mmol/l, non-HDL-C 5.4 mmol/l, fasting glucose 11.2 mmol/l, HbA1c 8.2%, creatinine 105 mmol/l eGFR 66.9 ml/min, and urine albuminuria 1+ (60 mg/day).
His repeat stress test showed strongly positive myocardial ischemia at moderate workload, his Duke Treadmill Score was 12.8, which corresponded to a 5-year mortality of 25%. His EuroScore was at least 15%.
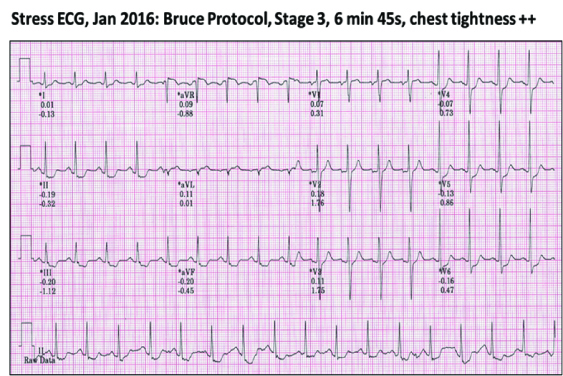
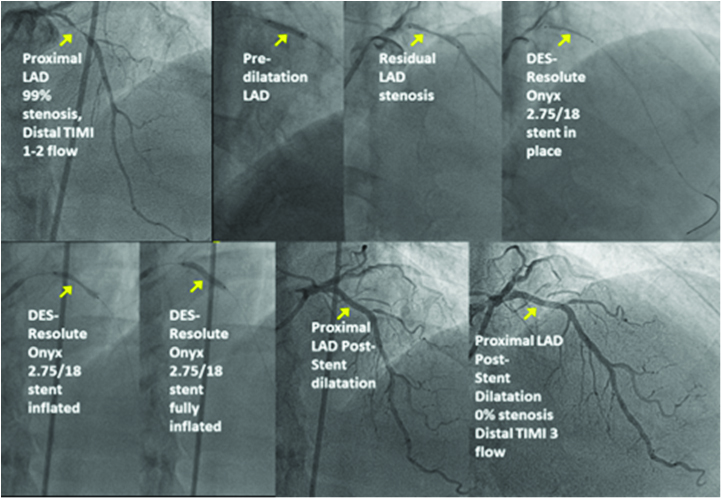
He consented to have another coronary angiogram with the view to ad hoc angioplasty when deemed necessary. This showed widely patent RCA and Circumflex stented segments, but the then minor mid-LAD lesion had now become 95-99% stenosed. He had uneventful successful angioplasty with an implanted Resolute Onyx 2.75mm/18mm stent.
He was discharged well, with modified medications and counselled strongly for adopting a better lifestyle modification programme, especially towards quitting smoking. His discharge medications now include: aspirin 100 mg daily, clopidogrel 75 mg daily, perindopril 5mg OD, amlodipine 5 mg OD, metformin SR 1000 mg x2 OD, empagliflozin 25 mg OD, gliclazide 120 mg OD, atorvastatin 40 mg OD, ezetimibe 10 mg OD. Atorvastatin 40 mg OD resulted in a LDL-C of 2.4 mmol/l, hence ezetimibe 10 mg OD was added. His glycemic control was difficult to achieve. Initially metformin 2g OD could not achieve the HbA1c to below 7.8%. Empagliflozin was titrated from 10 to 25 OD, to achieve a HbA1c of 7.2%. Gliclazide 60mg then 120 mg OD, was added to try to achieve target glycemic goals below 7%.
3 months later, his blood work-up showed the following: TC 3.3 mmol/l, TG 1.6 mmol/l, HDL-C 1.0 mmol/l, LDL-C 1.6 mmol/l, Non-HDL-C 2.3 mmol/l, HbA1C 6.8 %. There was no worsening of his renal function or albuminuria, His BP was now 135/78 mmHg; HR 65 bpm, and his BW 75 kg. His angina score has improved considerably to CCS class 0-1. His heart rate remained high in the 80s, so nebivolol 5 mg OD was added. He complained of some niggling chest discomfort still on exertion, especially on playing golf, for which trimetazidine 35 mg BID was added to good effect; he had quit smoking.
CASE DISCUSSION
RISK FACTOR MODIFICATION AND TARGETS From the early database of Framingham Heart Study, we know that when there are more risk factors clustered together in an individual, the risk for future cardiovascular events rises exponentially.7 E.g. a 45-year old hypertensive patient who has multiple risk factors such as diabetes, dyslipidemia, who smokes tobacco, or have an enlarged heart (ECG LVH) has 10 times the hazard rate of adverse CV outcomes, then when just having one factor alone (40 vs. 4 times the 10-year probability risk)!SDR, our patient presented to us at the age of 50 years, with multiple risk factors on top of acute coronary syndrome. This places him in the very high-risk category. Therefore, it behoves the physician to manage the patient comprehensively, by optimizing all his risk factors as effectively as possible. A recent state of the art review suggested a comprehensive holistic approach to medical therapy for long-term prevention of atherothrombosis following acute coronary syndrome.8
RISK STRATIFICATION
Clearly from a risk stratification viewpoint, this patient has very high risk for poor CV outcomes. He has had stable to unstable coronary artery disease with previous PCI, i.e. documented coronary artery disease, diabetes, trace proteinuria, is a current smoker, has hypertension, with still suboptimal blood LDL-C levels, and his 10-year ASCVD calculated risk score exceeds 20%. From guideline-directed management of dyslipidaemias, this is clearly ascribed as ‘Very High-Risk’ in both the ESC/EAS9 and ACC/AH10, and our own Malaysian guidelines.11A recent review of how we can manage our higher risk patients also refers to optimizing comprehensive management of all CV risk factors and co-morbidities.12 Aggressive LDL-cholesterol lowering has been associated with fewer recurrent major adverse cardiovascular events including death, fatal and non-fatal myocardial infarctions as well as stroke and hospitalization.8, 9

This patient’s estimated 10-year risk for CVD is well in excess of 20%. This means the strongest recommendations to manage all abnormal risk factors and comorbidities to target goals. Indeed, within that time frame, 9 years later he had recurrent unstable angina. This implies that we should aggressively use multiple medications per condition, to achieve all possible targets, for the best outcomes for the patient.
Let us get back to our patient, SDR. Some 9 years ago, he presented with unstable CAD, impaired fasting glucose, hypercholesterolaemia, who smokes and has hypertension. He had incident stent-angioplasties to his mid-distal RCA and mid-Circumflex arteries, with good initial results. But he defaulted close cardiac follow-up, resorting to somewhat less targeted pharmacotherapies that were maintained by his community pharmacist. Indeed, as predictable, he returned some 9 years later, with more aggressive progress of his condition: he was now a full-fledged diabetic, with poorly controlled risk factors, his LDL-C, his blood pressure, and his glycaemic control were all out of range of the guideline-mandated target goals. He still smoked. What are we to do now?
As physicians we have always been exhorted to treat our patients as holistically as possible, increasingly now with the remit to try and improve the natural history and potential progression of his ailments. In this case his multiple comorbidities have progressively become worse and uncontrolled.
CAD: CV RISK MANAGEMENT
Guidelines now offer a more targeted approach, based on comorbidities and concomitant risk factors that can be modified. For management of recurrent angina or equivalent, we must address relapses or progression of his coronary athero-thrombosis. Diagnostic coronary angiography is indicated for the worsening symptoms of his angina, proven by a strongly positive stress test (Class 1A, where the probability of significant disease is high >85%).13 Targeted revascularization for culprit lesions is also a Class IA indication for angina symptoms in chronic stable CAD.11Following revascularization i.e. with a drug-eluting stent, dual antithrombotic therapy is standard up to one full year in the context of unstable symptoms. Hence, aspirin and clopidogrel should be added to his armamentarium. Use of antiplatelet therapy has been shown to improve survival in patients with CAD, particularly so in patients with diabetes.14 In patients with stable ischemic heart disease (SIHD) treated with DAPT after drug-eluting stent (DES) implantation, P2Y12 inhibitor therapy with clopidogrel should be given for at least 6 months (Class I). In patients with unstable or acute coronary syndrome, routine DAPT is recommended after BMS or DES implantation, with P2Y12 inhibitor therapy (clopidogrel, prasugrel, or ticagrelor) given for at least 12 months (Class I).15
In the context of primary prevention however, in Japanese patients with diabetes, the JPAD-study did not find any significant reduction in CV events, despite there being non-significant but lower hazard ratios for atherosclerotic events (0.80, p=0.16).[16] Another Japanese study, Japanese Primary Prevention Project, studying the use of low-dose 100mg aspirin daily on patients 60-years and older with atherosclerotic risk factors, also did not show reduction of CV events for composite outcome of CV deaths, nonfatal stroke and, nonfatal myocardial infarction.17
But our use of DAPT for this patient is for secondary prevention. This has long been established to be beneficial. The 2001 PCICURE study showed that long-term treatment (8 months in this study) with clopidogrel and aspirin after PCI was associated with a lower rate of cardiovascular death, myocardial infarction, or any revascularisation (p=0.03), and of cardiovascular death or myocardial infarction (p=0.047). Overall (including events before and after PCI) there was a 31% reduction cardiovascular death or myocardial infarction (p=0.002).18
OPTIMAL MEDICAL THERAPY (OMT)
Indeed, since the patient continues to have symptoms despite his second revascularization, it behoves the physician to optimize the medical therapy as a viable alternative or an adjunct mode of controlling his symptoms, beyond just using antithrombotics. Optimal Medical Therapy (OMT) or Guideline Determined Medical Therapy (GDMT) should now be the standard of care for patients with chronic stable ischemic heart disease, particularly relevant after the publication of the 2007 COURAGE study19 and the recent 2015 extended COURAGE study.20
Manolis AJ et al., has suggested a tailored therapeutic approach that examines the haemodynamics, co-morbidities or risk factors, where myocardial anti-ischemic agents such as trimetazidine, ranolazine, ivabradine, nicorandil, may be used as first, second or third line in addition to beta-blockers, calcium channel blockers.21
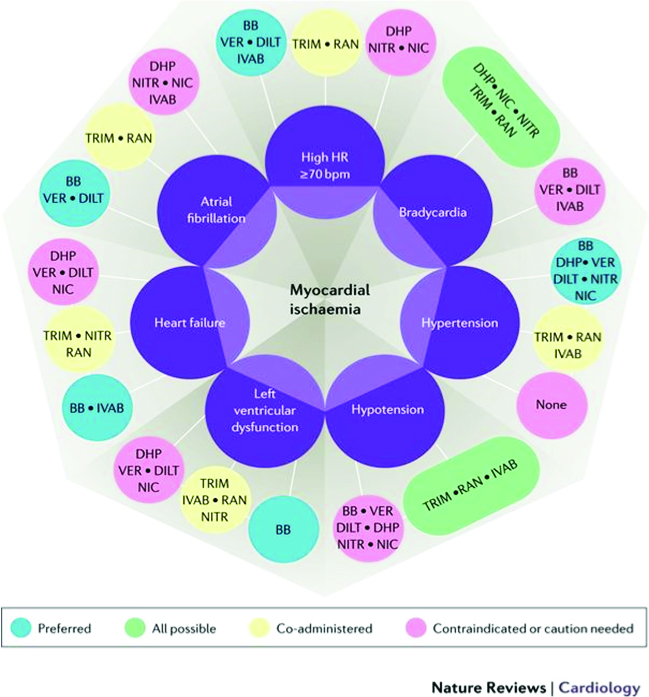
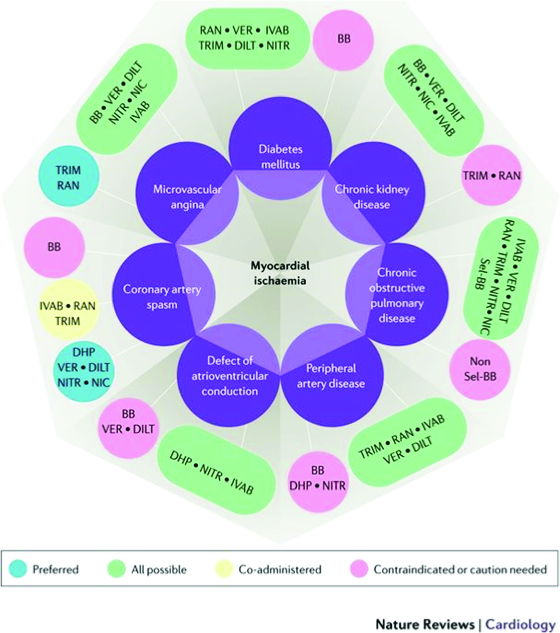
A more comprehensive approach called the Diamond approach has been proposed by Ferrari R et al, in a new consensus document first published in Nature Reviews: Cardiology, September 2017.22
This Diamond approach considers the comorbidities and underlying pathologies of the patient, with preferred choices of medication groups that can best be used for optimal outcomes and symptomatic relief. In the diamond itself, the darker green lines imply that these medications can be used in combination for synergistic effects and with few adverse interactions, the lighter green-blue or dotted lines show lesser evidence of added efficacy, while the red lines imply contraindications.

Using two other diamond charts for various conditions and comorbidities of the patient, allows us to make preferred as well as possible combination of drugs that have been shown to either improve outcomes, and/or to safely improve symptoms, with the minimum of adverse effects. Here the aquamarine blue capsules show the preferred options, the green capsules show the possible combinations, the yellow capsules show possible co-administration of the drugs, and the red-pink capsules show contraindication or caution to use.
Because our patient has several comorbidities, we can select from the various preferred combination choices. He has hypertension, diabetes, probable microvascular disease, thus we can choose from beta-blockers (with caution i.e. choosing those with minimal metabolic effects, e.g. nebivolol), calcium channel blockers, nitroglycerin, ranolazine, trimetazidine and/ or ivabradine if the heart rate remains high, and the angina remains refractory even after revascularization. This patient was prescribed amlodipine and perindopril for his hypertension and mild renal impairment (this was later changed to a fixed dose combination – Coveram 5/5 mg OD).
Nebivolol, a 3rd generation beta-blocker with nitric oxidepotentiating vasodilation effects23 and trimetazidine, an antiischemic metabolic agent (trimetazidine inhibits beta-oxidation of fatty acids by blocking long-chain 3-ketoacyl-CoA thiolase), which enhances glucose oxidation, was added for his ongoing residual angina.24
DIABETIC CONTROL AND CVOT REDUCTION
A recent review of how we should be managing type 2 diabetes (T2DM) better, has urged that “targeting multiple markers of CVD risk offers the best chance of improving CVD outcomes.”25 This review highlighted the importance of managing cardiovascular risk factors in patients with T2DM. There is now greater understanding that multiple factors associated with diabetes interact to result in premature and more aggressive cardiovascular disease, as the flow chart below shows.
Environmental factors and genetic predisposition lead to visceral obesity and inflammation that affects insulin resistance, glucose intolerance, which in turn cause endothelial dysfunction, abnormal haemostasis, low grade inflammation, hypercoagulable state and atherothrombosis. Hypertension and dyslipidemia are significant comorbidities that adversely impact renal/endothelial dysfunction leading to rapid progression of atherothrombosis and CVD.

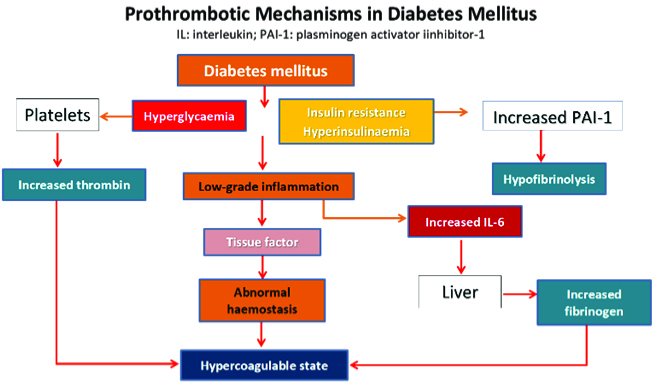
Thus, for diabetic patients, it is no longer enough to simply be concentrating on euglycemic control per se. We need to look beyond the microvascular and organ-based macrovascular pathologies that were dominating our management strategies. Most diabetic patients die from CV events, that are of course aggravated by comorbidities and kidney failure.
Thus, clinicians should provide guideline-directed medical therapy (e.g. lipid-lowering treatment, antiplatelet therapy) and glucose-lowering treatment like metformin. For a long time, the treatment of type 2 diabetes has not seen support in terms of longer-term favourable outcomes. Metformin was the only agent to show some small overall benefit, whereas a whole throve of hypoglycaemics including insulin, sulphonylureas, glinides, DPP4-inhibitors did not, despite improving glycemic control.
Intensive glycemic control of HbA1c to below 6.5% in fact increased CV events and mortality, despite decreasing microvascular complications. In the 6-year 10,251 strong ACCORD trial, HbA1c achieved in the intensive treatment group was 6.4 vs. 7.5% in the standard group. But there were 257 deaths in the intensive treatment arm compared with 203 in the standard treatment group (hazard ratio, 1.22; 95% CI, 1.01 to 1.46; P = 0.04)—a difference of 54 deaths, or 3 per 1,000 participants each year.26 Thus, there is uncertainty as to whether intensive glycemic control is beneficial in the long term.
However, the ADVANCE study did not show signals of increased mortality from intensive therapy for diabetes.[27] This 5-year 11,140 patients ADVANCE study, showed that tighter control slightly improves microvascular outcomes, 18.1% vs. 20.0%, HR 0.90. Incidence of nephropathy was reduced by 21%, i.e. 4.1% vs. 5.2% (HR 0.79, p = 0.006). But there was no significant reduction in macrovascular (HR 0.94, p = 0.32) or cardiovascular outcomes (HR 0.88, p = 0.12), or death from any cause (HR 0.93, p = 0.28). There were more severe hypoglycaemic complications in the intensive arm, 2.7% vs. 1.5%, HR 1.86, p < 0.001).
The smaller 1791 military patients VADT study 7.5 years, showed that tighter glycemic control of HBA1c of 6.9% vs. 8.4%, led to a nonsignificant reduction of primary outcomes, HR 0.88, p = 0.14), with no difference in microvascular outcomes as well. The study concluded that intensive glucose control in patients with poorly controlled type 2 diabetes had no significant effect on the rates of major cardiovascular events, death, or microvascular complications, except for slower progression of albuminuria (P = 0.01).28
The 2008 Canadian Diabetes Association clinical practice guidelines suggested that an HbA1c < 7% will reduce microvascular complications. But to further reduce the risk of nephropathy, an HbA1c < 6.5% is beneficial. For reducing macrovascular disease, this is less clear. The guidelines suggest that the most worthwhile approach is to target an HbA1c < 7% and begin a multifaceted cardiovascular risk-reduction approach as early as possible.29
Recent post-hoc analyses of the ACCORD study suggest that there is a U-shape curve of mortality risk associated with too intensive or low HbA1c-level treatment strategies.30, 31
NOVEL HYPOGLYCAEMIC AGENTS WITH CVOT: SGLT2-I & GLP1-RA
Recently the American College of Cardiology released an updated consensus on how to manage the high-risk diabetic patient with ASCVD. ACC recommends a more comprehensive decision algorithm pathway.32
In brief, as clinicians we are exhorted to adhere to guidelines as closely as possible to achieve the best clinical outcomes for our patients.
In the past few years, there have been a cluster of successful CVOT studies that used novel hypoglycaemic agents, particularly the oral SGLT2-inhibitors and the injectable GLP-1 Receptor-Antagonists.
Therefore, we should consider adding an SGLT2 inhibitor (empagliflozin is the preferred agent) or a GLP-1RA (liraglutide is preferred) with proven CV benefit following a discussion with the patient. This is as outlined below.29 The preferred choice for either or both agents is based on the study outcomes of clearer superiority.
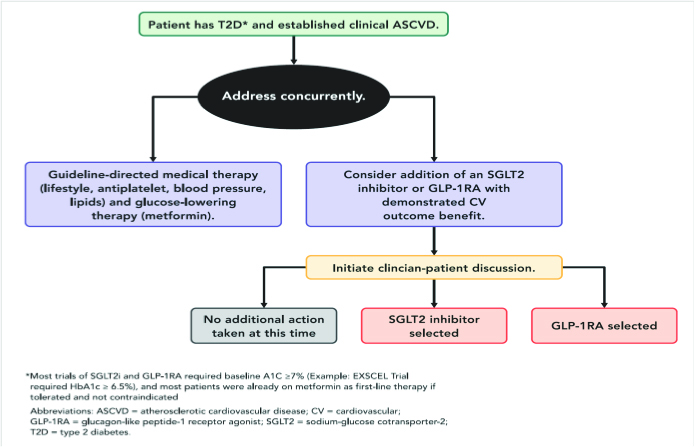
In September 2015, the EMPA-REG OUTCOME study was terminated early.33 This showed for the first time that treating type 2 diabetes with empagliflozin, in a high-risk post-CAD cohort of patients, was associated with significantly better CV outcomes. In this study of 7020 patients treated over a median 3.1 years, the primary outcome was achieved with a hazard ratio in the empagliflozin group of 0.86; 95% CI, 0.74 – 0.99; P = 0.04 for superiority). There were in the empagliflozin group, significantly lower rates of death from cardiovascular causes of 3.7%, vs. 5.9% in the placebo group; i.e. 38% relative risk reduction; for hospitalization for heart failure 2.7% vs. 4.1%, respectively; 35% relative risk reduction, and death from any cause 5.7% and 8.3%, respectively; 32% relative risk reduction.
The CANVAS study using another SGLT2-inhibitor canagliflozin also showed superior endpoints but with some hints of slight increase in limb amputation risk.34 Primary outcome was lower with canagliflozin than with placebo i.e. 26.9 vs. 31.5 participants per 1000 patient-years; hazard ratio, 0.86; 95% CI, 0.75 to 0.97; P<0.001 for noninferiority; P=0.02 for superiority. Canagliflozin also confers benefits on the progression of albuminuria (hazard ratio, 0.73; 95% CI, 0.67 to 0.79) with the composite outcome of a sustained 40% reduction in the estimated glomerular filtration rate, the need for renal-replacement therapy, or death from renal causes (hazard ratio, 0.60; 95% CI, 0.47 to 0.77). One serious adverse reaction was an increased risk of amputation (6.3 vs. 3.4 participants per 1000 patient-years; hazard ratio, 1.97; 95% CI, 1.41 to 2.75); the amputations were primarily at the level of the toe or metatarsal.
The most recent positive study, the DECLARE-TIMI study was announced at the AHA scientific Sessions in November 2018.[35] 17,160 patients were studied, 10,186 of which did not have atherosclerotic cardiovascular disease. They were followed for a median of 4.2 years. In primary efficacy analyses, dapagliflozin did not show a lower rate of MACE (8.8% in the dapagliflozin group and 9.4% in the placebo group; hazard ratio, 0.93; 95% CI, 0.84 to 1.03; P = 0.17). But there was lower rate of cardiovascular death or hospitalization for heart failure (4.9% vs. 5.8%; hazard ratio, 0.83; 95% CI, 0.73 to 0.95; P = 0.005), particularly a lower rate of hospitalization for heart failure (hazard ratio, 0.73; 95% CI, 0.61 to 0.88). However, there was no between-group difference in cardiovascular death (hazard ratio, 0.98; 95% CI, 0.82 to 1.17). Renal events were lower in the dapagliflozin group (4.3%) than the placebo group (5.6%) (hazard ratio, 0.76; 95% CI, 0.67 to 0.87). But death from any cause was no different (hazard ratio, 0.93; 95% and the rate of serious genital infections, higher (0.9% vs. 0.1%, P < 0.001).
However, in this predominantly primary prevention study, the CV outcomes results were not as robust as compared with the EMPA-REG which was 99% a secondary prevention study.
The injectable anti-hyperglycaemic agent Liraglutide, a glucagon-like peptide-1 receptor antagonist, in the LEADERS study also showed beneficial CV outcomes, especially CV deaths and all deaths.36 9,340 patients were studied for 3.8 years. Primary outcome was significant the liraglutide group [13.0%] vs. placebo group [14.9%] with a hazard ratio of 0.87; 95% CI, 0.78 – 0.97; P<0.001 for noninferiority; P=0.01 for superiority. Fewer patients died from CV causes in the liraglutide group [4.7%] than in the placebo group [6.0%], a hazard ratio, 0.78; 95% CI, 0.66 - 0.93; P=0.007. Death rate from any cause was also lower in the liraglutide group [8.2%] than in the placebo group [9.6%], a hazard ratio, 0.85; 95% CI, 0.74 to 0.97; P=0.02.
Another injectable GLP-1-RA, semaglutide was shown to have similar outcomes in the SUSTAIN-6 study.37 In this study, 2,735 of the patients (83.0%) had established CV disease, chronic kidney disease, or both. Primary outcome was 6.6% in the semaglutide group vs. 8.9% in the placebo group (hazard ratio HR, 0.74; 95% CI, 0.58 to 0.95; P<0.001 for noninferiority). Nonfatal myocardial infarction occurred in 2.9% of the patients receiving semaglutide vs. 3.9% in those receiving placebo (HR, 0.74; 95% CI, 0.51 to 1.08; P = 0.12); nonfatal stroke occurred in 1.6% and 2.7%, respectively (HR, 0.61; 95% CI, 0.38 to 0.99; P = 0.04). The difference here is that semaglutide use was not associated with reduction in death rates from cardiovascular causes. Retinal complications (vitreous haemorrhage, blindness, etc.) were significantly higher with semaglutide (HR, 1.76; 95% CI, 1.11 to 2.78; P = 0.02).
With these novel drugs showing consistent favourable CV outcomes, it obliges physicians to seriously consider adding these agents to our frontline evidence-based armamentarium of therapies for our very high CV risk patients. How then do we choose?
The 2018 ACC expert consensus recently describes in detail the decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease.30 This new approach is also echoed by the American Diabetic Association and European Association for the Study of Diabetes, which has produced some clear algorithms for clinical practice.38
ARE MULTIFACTORIAL INTERVENTIONS BENEFICIAL?
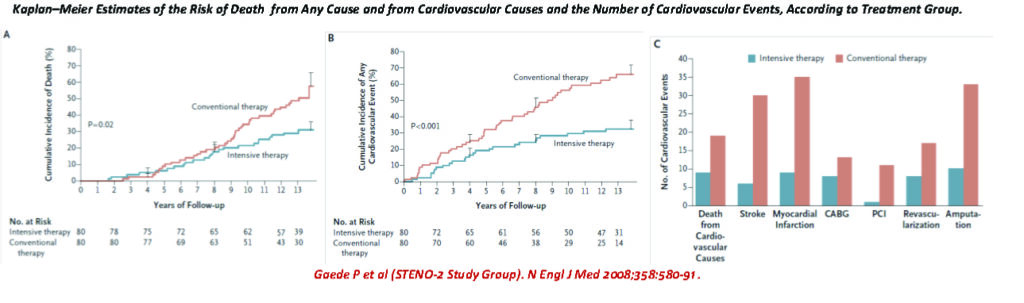
One of the earliest studies to examine this premise, is the STENO-2 study,39 where a small cohort (80 in each arm) of type 2 diabetic patients with microalbuminuria, were studied to compare targeted, intensified, multifactorial intervention vs. conventional treatment, for modifiable CV risk factors. The mean age was 55.1 years and the mean follow-up was 7.8 years. A second follow-up review was performed 5-years later.40
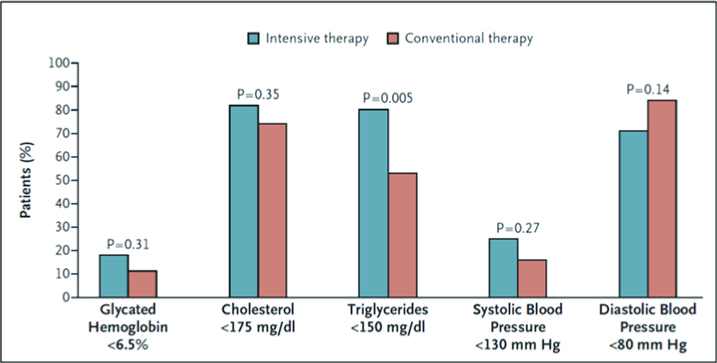
Interventions included targeting SBP to <130mmHg, DBP to <80mmHg, HbA1c to <6.5%, total cholesterol to <175mg/dl, triglycerides to <150mg/dl, with add-on ACE-inhibitors, and use of aspirin in those with ASCVD.
The intensively targeted cohort was able to achieve these goals more consistently vs. those that were managed by conventional therapy.

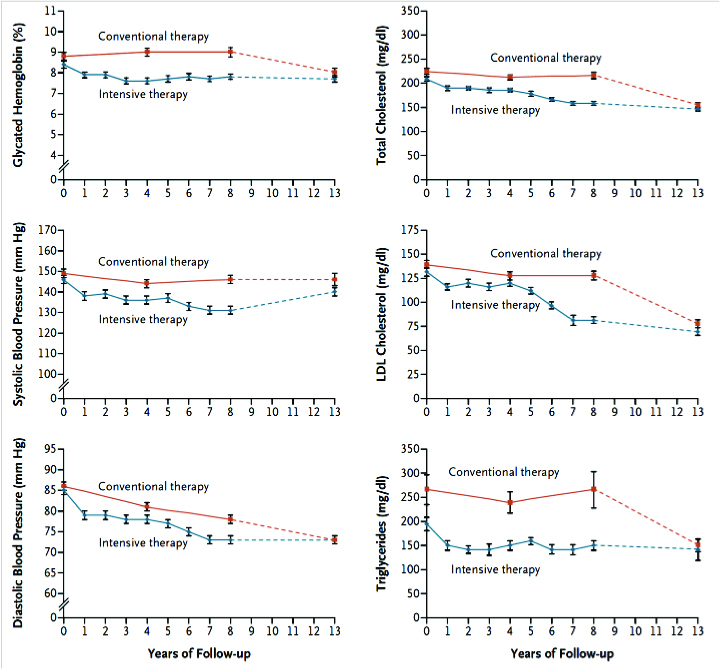
However, on further follow-up 5-years later (mean follow-up of 13.3 years), the benefits were by and large attenuated, as more of the conventional therapy cohort caught up in terms of achieving target goals when compared with the intensive cohort.
The longer-term CV outcomes also became attenuated and converged, as well. But multivariate analysis showed that the better survival curves for deaths and CV events, were indeed persistent in the intensive therapy group vs. conventional group.
Patients receiving intensive therapy had a significantly lower risk of cardiovascular disease (hazard ratio, 0.47; 95% CI, 0.24 to 0.73), nephropathy (hazard ratio, 0.39; 95% CI, 0.17 to 0.87), retinopathy (hazard ratio, 0.42; 95 % CI, 0.21 to 0.86), and autonomic neuropathy (hazard ratio, 0.37; 95 % CI, 0.18 to 0.79).
The extended STENO-2 study37 also concluded that a target driven, long-term, intensified intervention aimed at multiple risk factors in patients with type 2 diabetes and micro-albuminuria reduces the risk of cardiovascular and microvascular events by about 50 percent.
From the Kaplan-Meier Survival curves it appears that intensive multifactorial CV risk reduction intervention have better long term benefits, compared with conventional therapy, in diabetic patients with microalbuminuria.
Since then there have been many attempts at more rigorous intensive approach to treating the diabetic patient with multiple comorbidities, with variable results.
A recent population wide Swedish National Diabetes Register study that investigated 271,174 patients with diabetes, found that when 5 concomitant risk factors (elevated glycated haemoglobin level, elevated low-density lipoprotein cholesterol level, albuminuria, smoking, and elevated blood pressure) are managed well to within target ranges, there were consistently better outcomes over a median duration of 5.7 years.41
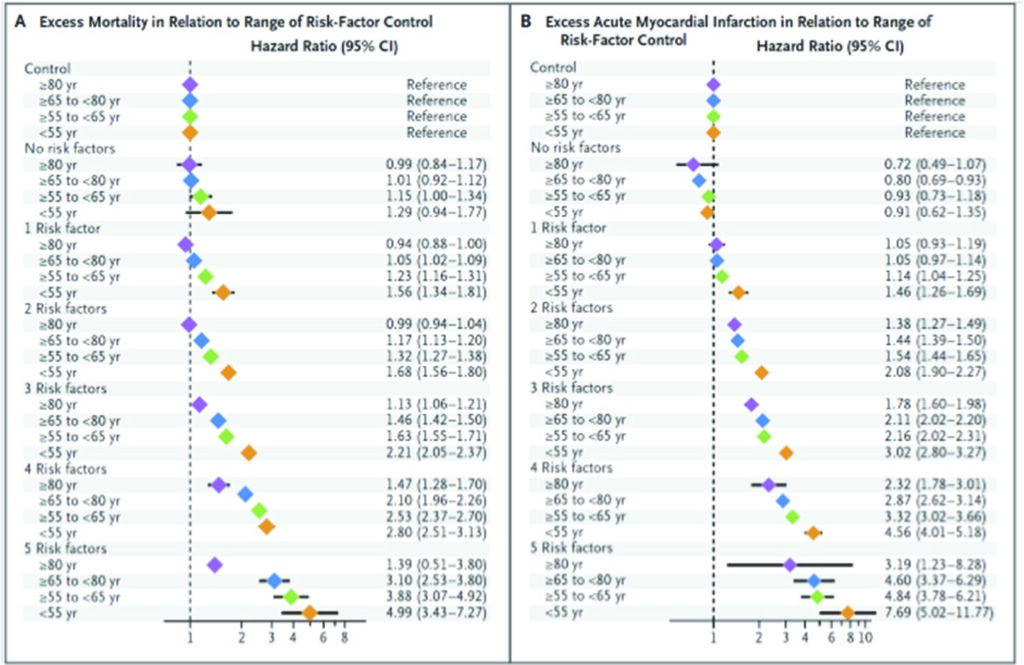
For those patients with diabetes who had all five variables within target ranges, there was no difference in the hazard ratio for death from any cause, as compared with controls, was 1.06 (95% confidence interval [CI], 1.00 to 1.12). However, the hazard ratio (HR) for acute myocardial infarction was lower at 0.84 (95% CI, 0.75 to 0.93), and the HR for stroke was 0.95 (95% CI, 0.84 to 1.07). This compares with hazard ratios that were extremely high if these risk factors were not within controlled target ranges: i.e. for overall mortality HR the those with all 5 risk factors was 3.55 (CI 2.92-4.32); for acute myocardial infarction, HR 5.28 (CI 4.48 – 6.21); for stroke, HR 3.58 (CI 2.70 – 4.76) and heart failure, HR 5.64 (CI 4.68 – 6.80).
A logarithmic escalation of hazard ratios from 1 to 5 risk factors outside the target range is seen, suggesting that with more risk factors not optimally controlled, the hazard ratios for cardiovascular events rise exponentially. The hazard ratios for these risk factors appear to be attenuated with age.42
Importantly, the risk of hospitalization for heart failure was consistently higher among patients with diabetes than among controls (hazard ratio, 1.45; 95% CI, 1.34 to 1.57). Patients with glycated haemoglobin level falling outside the target range, had the strongest predictor of stroke and acute myocardial infarction; whilst smoking was the strongest predictor of death. The most important conclusion is that diabetic patients who had five risk factor variables within the target ranges, appeared to have little or no excess risk of death, myocardial infarction, or stroke, as compared with the general population.38
While this Swedish population-based research did not specifically study the effects of treatment, the association of identified risk factors with CV events, comparing people within and outside the accepted norms of target ranges, compellingly implies that in managing our patients with multiple comorbidities, controlling these factors to these known goals, can substantially reduce their risks from future incidences of major cardiovascular events and death.
Another recent review (PROSPERO) by Seidu et al.43 showed that multifactorial interventions were beneficial. A total of 19 RCTs were analysed: 16 examined non-fatal MI (n = 79,595), 14 non-fatal stroke (n = 78,568), 18 cardiovascular mortality (n = 83,938) and 18 all-cause mortality (n = 84,266). Results were inconsistent.
Compared with standard care, intensive glycemic therapy did reduce the risk of non-fatal MI (RR = 0.90, 95% CI 0.83-0.96), but not non-fatal stroke (RR 0.96, 95% CI 0.86-1.07), CV mortality (RR 1.00, 95% CI 0.90-1.11) or all-cause mortality (RR 1.00, 95% CI 0.94-1.06); whereas multifactorial interventions alone also reduced non-fatal stroke (RR 0.53, 95% CI 0.32-0.0.87) but not non-fatal MI (RR 0.66, 95% CI 0.38-1.03), CV mortality (RR 0.72, 95% CI 0.46-1.14) or all-cause mortality (RR 0.82, 95% CI 0.64- 1.05).
They concluded that where the average 10-year CVD risk is high, i.e. above 6.3%, combining intensive glucose-lowering plus multifactorial interventions can result in the desired beneficial effect of reducing CVD mortality in populations.
PROBLEMS ASSOCIATED WITH POLYPHARMACY IN MULTIMORBIDITY PATIENTS
Polypharmacy in people with multimorbidity is based on the premise that more medicines are needed to reduce the risk of future morbidity and mortality in specific health conditions. However, as pointed out by the NICE guidance44, “The absolute benefit made by each additional medicine is likely to reduce when a person is taking multiple preventative medicines; often referred to as the law of diminishing returns. However, the risk of harms is likely to increase with additional medicines being taken.”
The King’s Fund report (2013)45has proposed the classification of polypharmacy as either ‘Appropriate’ or ‘Problematic’. This report recognises that not all polypharmacy is inappropriate. Appropriate polypharmacy is defined as “Prescribing for a person for complex conditions or for multiple conditions in circumstances where medicines use has been optimised and where the medicines are prescribed according to best evidence.” Problematic polypharmacy is defined as “The prescribing of multiple medicines inappropriately, or where the intended benefit of the medicines is not realised.”
Therefore, while we advocate rational and appropriate polypharmacy in multimorbidity patients, we must be wary of the potential to increase adverse effects and drug-drug interactions, i.e. the potential occurrence of occasional worsening or aggravation of organ dysfunction and failure.
Indeed, one of the strongest critiques against polypharmacy is in the elderly age group, where the potential for harm may exceed the benefits. But how are we to determine when the age barrier kicks in, to negate the benefits of holistic and aggressive management of multiple comorbidities?
Boyd et al, in a 2005 paper, discussed this particular conundrum in the older patients with multiple comorbidities.46 They concluded that “most CPGs do not modify or discuss the applicability of their recommendations for the older patients with multiple comorbidities, nor comment on the burden, short- and long-term goals and quality of underlying scientific evidence. There is little guidance on how to incorporate patient preferences into treatment plans.” Of course, any complicated non pharmacological regimen added to 10-12 medications, can also confound the ageing patients, where adverse interactions between drugs and disease could occur.
They also cautioned against “the use of CPGs as the basis for pay-for-performance initiatives that focus on specific treatments for single diseases”, stressing that this “may be particularly unsuited to the care of older individuals with multiple chronic diseases.” They advocate that for quality improvement, payfor- performance initiatives should be designed to improve the quality of care for older patients with multiple comorbidities.
One critical first step is to improve clinical research “to define measures of the quality of care needed by this population, including care coordination, education, empowerment for self-management, and shared decision-making based on the individual circumstances of older patients.” For the relatively younger (<65 years old) middle-aged patient, polypharmacy for multiple comorbidities may be optimum, but this may not apply to the older patient. Importantly, we should be guided by more robust evidence of benefits, i.e. outcomes in the longer term, and not just short-term attainment of isolated goals or targets. A recent editorial comment by van Weel and Schellevis, urge physicians to be more wary when dealing with patients with multiple comorbidities.47
We must choose a patient-centred rather than a diseaseoriented approach. We need to address individual needs while integrating various disease perspectives; this is what determines its effectiveness. This is even more critical when we are dealing with the patient from a family or general practice perspective, where we need to factor in cost constraints, patient dynamics and infirmity.48
Schellevis FG, et al., divided comorbidity into four categories:49
1. causal, diseases with a common pathophysiology;
2. complicating, disease-specific complicating morbidity;
3. concurrent, co-existing chronic morbidity without any known causal relation to the index disease; and
4. intercurrent, referring to interacting acute illness, usually limited in time.
The first 3 categories are what is relevant to our discussion here. This concept is interesting because, it has implications for patients’ care. “When the comorbidity is causally related to or is a complication of the index disease, disease-specific guidelines can be used to direct management.” Nevertheless, guidelines must include adequate information on health risks associated with the index condition.
Better guidelines can enhance proactive management of illness. However, their development must include patients with wider spectrum of comorbidities in the randomised trials.50Currently, many disease-specific guidelines are problematic and confusing when there is concurrent morbidity, associated with ageing related diseases and frailty. Geriatricians such as Fried et al,51 has cautioned that the interaction of diseases and their pharmacological “management require more complex and individualized care, than simply the sum of separate guideline components.” Instead of developing newer more complex guidelines that include all possible combinations of diseases, they advocate a simpler, more “holistic patient-centred approach, ensuring continuity of care and integrating the patients’ biopsychosocial domains.”
We should be prudently cautious and monitor the patient closely with regards adverse vs. beneficial effects, considering age, renal and hepatic function and drug-drug interaction, particularly on whether these enhance or decrease drug activation, metabolism, half-lives and bioavailability patterns. We therefore welcome more studies that combine therapeutic approaches or more reports on the natural course of patients with multiple comorbidities.
CONCLUSION
Therefore, in this case illustration of a middle-aged diabetic patient with multiple CV risks, it is incumbent upon the physician to be as rigorous in managing these risk factors according to current accepted evidence-based guidelines.
We need to be aggressive and target-oriented and treat all comorbidities and risk factors to goal. Only then are we serving in our patients’ best interest and thus, offer them the best possible long-term outcomes.
Polypharmacy in these patient groups is therefore not overtreatment, but obligatory and appropriate management of the patient as a whole. But pill counts can still be daunting and make drug adherence confusing to the patient. Thus, there are some major efforts made towards reducing the pill count for the patient with multiple comorbidities. Fixed dose combination pills can help reduce the pill count to improve patients’ medication adherence. Bangalore S et al,52 showed from meta-analysis of 9 studies that fixed-dose combination resulted in a 26% decrease in non-compliance compared with free-drug component regimen (pooled relative risk [RR] 0.74; 95% CI 0.69-0.80; P <.0001).
A more recent multinational study (SPACE Collaboration on polypill management of hypertension and hypercholesterolemia)[53] showed that the polypill arm had higher adherence to combination therapy (80% vs. 50%, RR 1.58; 95% CI, 1.32 to 1.90; p < 0.001), lower SBP (−2.5 mmHg; 95% CI, −4.5 to −0.4; p = 0.02) with better outcomes of lower LDLcholesterol (−0.1 mmol/L; 95% CI, −0.2 to 0.0; p = 0.04).
In this very high-risk, relatively young patient (SDR) with multiple co-morbidities, we sought to improve his medication adherence by using (Co-Plavix (aspirin + clopidogrel), Coveram (perindopril + amlodipine), Atozet (atorvastatin 40mg + ezetimibe 10 mg).
Triple antihyperglycemic agents54 with empagliflozin 10 and 25 mg for 24 weeks as add-on to metformin plus sulfonylurea has been shown to improve glycemic control, weight, and systolic blood pressure and were well tolerated. Possibly later fixed dose combination (Synjardy, empagliflozin + metformin, when this becomes available), may be used to help reduce his pill burden further, thereby improving his medication adherence and compliance.
As physicians we must move towards greater holistic management of the whole patient versus the frequently unflattering snide remarks that we practice in blinkered silos and disciplines.
There are several trajectories of ill-health, as illustrated in the charts below.55 Chris Feudtner, a paediatrician and ethicist, posits that there are several trajectories of chronic ill health and dying. He is credited with the charts below. We obviously would like to prevent any sudden death as in case scenario A. Neither should we wait for the scourge of chronic disease to affect the quality of life so seriously that the patient is fragile and poorly as in case D. Hopefully, as evidence-based physicians we can offer our patients either trajectory B or failing which, at least C.
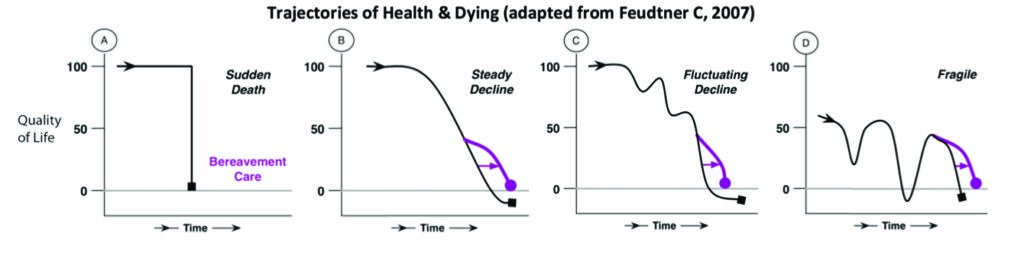
Increasingly physicians are faced with accepting the fact that all our patients would eventually die. The question is not if, but when. With a chronic disease entity like ASCVD and especially those with multiple co-morbidities, we are faced with having to encounter recurrent episodes of events, ill-health and eventually dying.
How we manage their illness and how we can prolong the process of decay and deterioration is what makes us humane and successful as empathetic healers. We postpone the inevitable falling apart of our human body, our inexorable entropy.
Let’s be focused on blunting the inexorable decline in health and quality of life of our ASCVD patients, by prolonging their health as best as possible. We can and are getting better at this. We have a growing cache of strategies and therapies that have helped many if not most of patients to lead not just longer lives, but with greater quality of life, as well.
As physicians we must assist every individual patient to “rage mightily against the dying of the light”, to fight against the inexorable ageing entropy of the human body, no matter that fact that we will all die someday. We must choose to offer and extend good quality life for as long as is prudent and possible.
“It has become, in my view, a bit too trendy to regard the acceptance of death as something tantamount to intrinsic dignity. Of course, I agree… there is a time to love and a time to die— and when my skein runs out I hope to face the end calmly and in my own way. For most situations, however, I prefer the more martial view that death is the ultimate enemy—and I find nothing reproachable in those who rage mightily against the dying of the light.” ~Stephen Jay Gould, palaeontologist, evolutionary biologist.56
But clearly, we must not forget the relentless march of ageing and its attendant entropic breakdown of our organs and tissues, we will fall apart…57 We too, must not overestimate the value of modern medicines, which some have liken to the ancient cultural spirits and gods of yesteryears, sometimes fraught with offer of false promises and its underestimated or delayed hazards.58
“There is no single, common cellular mechanism to the ageing process… The process is gradual and unrelenting… We just fall apart.” ~ Dr Felix A. Silverstone, former senior geriatrician, Parker Jewish Institute in New York, died 98 years, April 30, 201852
“Prescription drugs are both wonderful and dangerous. They allow us to live longer, they allow us to suffer less, but they may also offer false promises of happiness and health and immortality that they cannot possibly deliver. In this they are more like the spirits and gods of other cultures than we care to believe.” ~ Freeman S, Critchley D and Lee L. ‘Cradle to Grave’. In Wynants M (ed.). In Sickness and in Health: The Future of Medicine – Added Value and Global Access.58
REFERENCES
1. Richard Smith. “Diagnose, treat, and cure” is largely dead. http://blogs. bmj.com/bmj/2015/06/15/richard-smith-diagnose-treat-and-cure-is-largely-dead/. Accessed 24 Nov 2018.
2. Sharma A, Green JB, Dunning A, et al (TECOS Study group). Causes of Death in a Contemporary Cohort of Patients With Type 2 Diabetes and Atherosclerotic Cardiovascular Disease: Insights From the TECOS Trial. Diabetes Care 2017 Dec; 40(12): 1763-1770. CrossRef Pubmed
3. Chen L., Pei J-H, Kuang J, et al. Effect of lifestyle intervention in patients with type 2 diabetes: A meta-analysis. Metabolism. 2015;64:338-347. http://dx.doi.org/10.1016/j.metabol.2014.10.018. CrossRef
4. Koivula RW, Tornberg AB, Franks PW. Exercise and diabetes-related cardiovascular disease: systematic review of published evidence from observational studies and clinical trials. Curr Diab Rep 2013;13(3):372–80. Pubmed
5. Brand T, Pischke CR, Steenbock B, Schoenbach J, Poettgen S, Samkange- Zeeb F, et al. What works in community-based interventions promoting physical activity and healthy eating? A review of reviews. Int J Environ Res Public Health 2014;11(6):5866–88. CrossRef Pubmed
6. Balducci S, Zanuso S, Nicolucci A, De Feo P, Cavallo S, Cardelli P, et al. Effect of an intensive exercise intervention strategy on modifiable cardiovascular risk factors in subjects with type 2 diabetes mellitus: a randomized controlled trial: the Italian Diabetes and Exercise Study (IDES). Arch Intern Med 2010;170: 1794–803. CrossRef Pubmed
7. Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13:3S–10S. Pubmed
8. Gallone G, Baldetti L, Pagnesi M, et al. Medical therapy for long-term prevention of atherothrombosis following an acute coronary syndrome. JACC state-of-the-Art Review. J Am Coll Cardiol 2018;72:2886-903. CrossRef Pubmed
9. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J (2016) 37, 2999–3058. doi:10.1093/eurheartj/ehw272. CrossRef Pubmed
10. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018; DOI: 10.1161/CIR.0000000000000625. CrossRef
11. Robaayah Z, Jeyamalar R, et al. Clinical Practice Guidelines on Management of Dyslipidaemia 2017, 5th Edition. July 2017. MOH/P/ PAK/344.17(GU)
12. Jeyamalar Rajadurai, Wan Azman Wan Ahmad, Hapizah Nawawi, Choo Gim Hooi, Ng Wai Kiat, Rosli Mohd Ali, Al Fazir Omar, Sazzli Kasim, Oteh Maskon, David Kwang Leng Quek. Updates in the management of Dyslipidaemia in the high and very high risk individual for CV risk reduction. Med J Malaysia 2018;73(3):154-162. Pubmed
13. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardio-Thorac Surg 46 (2014) 517–592. CrossRef Pubmed
14. Harpaz D, et al. Am J Med 1998;105:494-499. Pubmed
15. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68(10):1082-1115. CrossRef Pubmed
16. Ogawa H, Nakayama M, Uemura S, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: A randomized controlled trial. JAMA. 2008;300:2134-2141. CrossRef Pubmed
17. Ikeda Y, Shimada K, Teramoto T, et al. (for JPPP). Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. JAMA 2014;312(23):2510-20. CrossRef Pubmed
18. Mehta SR, Yusuf S, Peter RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001 Aug 18;358(9281):527-33. CrossRef Pubmed
19. Boden WE, O’Rourke RA, Teo KK, et al. for the COURAGE Trial Research Group. Optimal Medical Therapy with or without PCI for Stable Coronary Disease. N Engl J Med 2007;356:1503-1516. doi: 10.1056/NEJMoa070829. CrossRef Pubmed
20. Sedis SP, Martigan PM, Teo KK, et al. for the COURAGE Trial Research Group. Effect of PCI on long-term survival in patients with stable ischemic heart disease. N Engl J Med 2015;373:1937-46. CrossRef Pubmed
21. Manolis AJ, et al. Medical treatment of stable angina: A tailored therapeutic approach. International J Cardiol 2016; 220:445-453. doi: 10.1016/j.ijcard. 2016.06.150. CrossRef Pubmed
22. Roberto Ferrari, Paolo G. Camici, Filippo Crea, et al. Expert consensus document: A ‘diamond’ approach to personalized treatment of angina. Nature Reviews Cardiology, 2018;15:120-132. doi: 10.1038/nrcardio. 2017.131 CrossRef Pubmed
23. Weiss R. Nebivolol: a novel beta-blocker with nitric oxide-induced vasodilatation. Vasc Health Risk Manag. 2006;2(3):303–8. Pubmed
24. Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ. Res. 2000;86 (5):580–8. doi:10.1161/01. RES.86.5.580. CrossRef Pubmed
25. Iciar Martín-Timón, Cristina Sevillano-Collantes, Juan José Marín-Peñalver, Francisco Javier del Cañizo-Gómez. Management of Cardiovascular Risk Factors in Type 2 Diabetes Mellitus Patients. Eur Med J. 2016;1[4]:89-97.
26. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study Group; Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358(24):2545-59. CrossRef Pubmed
27. ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358(24):2560-72. CrossRef Pubmed
28. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360(2):129-139. CrossRef Pubmed
29. Canadian Diabetes Association. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 2008;32(Suppl 1):1-215.
30. Riddle MC, Ambrosius WT, Brillon DJ, et al. Epidemiologic Relationships Between A1C and All-Cause Mortality During a Median 3.4-Year Follow-up of Glycemic Treatment in the ACCORD Trial. Diabetes Care 2010;33:983-990. CrossRef Pubmed
31. Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study. Lancet 2010;375:481-489. CrossRef Pubmed
32. Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL Jr, Rastogi Kalyani R, Kosiborod M, Magwire ML, Morris PB, Sperling LS. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;72:3200–23. CrossRef Pubmed
33. Zinman B, Wanner C, Lachin JM, Fitchett D, et al. for EMPA-REG OUTCOMES Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015 Nov 26;373(22):2117-28. doi: 10.1056/NEJMoa1504720. CrossRef Pubmed
34. Neal B, Perkovic V, Mahaffey KW, et al. for CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/ NEJMoa1611925. CrossRef Pubmed
35. Wiviott SD, Raz I, Bonaca MP, et al., for the DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2018, prepublication November 10, 2018, at NEJM.org. doi: 10.1056/NEJMoa1812389. CrossRef Pubmed
36. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–22. CrossRef Pubmed
37. Marso SP, Bain SC, M.D., Consoli A, et al., for the SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2016; 375:1834-1844. doi: 10.1056/ NEJMoa1607141. CrossRef Pubmed
38. Davies MJ, A’Allesio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care 2018;41:2669–2701. CrossRef Pubmed
39. Gæde P, Vedel P, Larsen N. et al (STENO-2 Study). Multifactorial Intervention and Cardiovascular Disease in Patients with Type 2 Diabetes. N Engl J Med 2003;348:383-93. CrossRef Pubmed
40. Gæde P, Lund-Andersen H, Parving H-H, Pedersen O (STENO-2 Study). Effect of a Multifactorial Intervention on Mortality in Type 2 Diabetes. N Engl J Med 2008;358:580-91. CrossRef Pubmed
41. Rawshani A, Rawshani A, Franzén S, et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med 2018; 379:633-644. doi: 10.1056/NEJMoa1800256 CrossRef Pubmed
42. Supplement to: Rawshani Aidin, Rawshani Araz, Franzén S, et al. Risk factors, mortality, and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2018;379:633-44. doi: 10.1056/NEJMoa1800256. CrossRef Pubmed
43. Seidu S, Achana FA, Gray LJ, Davies MJ, Khunti K. Effects of glucoselowering and multifactorial interventions on cardiovascular and mortality outcomes: a meta-analysis of randomized control trials. Diabet Med. 2016 Mar;33(3):280-9. CrossRef Pubmed
44. National Insitute for Health and Care Excellence (NICE). Multimorbidity and polypharmacy. 16 Jan 2017. nice.org.uk/guidance/ktt18.
45. Polypharmacy and medicines optimisation: making it safe and sound. The King’s Fund 2013.
46. Boyd CM, Darer J, Boult C, et al. Clinical Practice Guidelines and Quality of Care for Older Patients With Multiple Comorbid Diseases – Implications for Pay for Performance. JAMA. 2005;294:716-724.CrossRef Pubmed
47. Van Weel C, Schellevis FG. Comorbidity and guidelines: conflicting interests. Lancet 2006; 367:550-551. CrossRef Pubmed
48. Starfield B, Lemke KW, Bernhardt T, Foldes SS, Forrest CB, Weiner JP. Comorbidity: implications for the importance of primary care in ‘case’ management. Ann Fam Med 2003;1:8–14. CrossRef Pubmed
49. Schellevis FG, van de Lisdonk EH, van der Velden J, Hoogbergen SHJL, van Eijk JTM, van Weel C. Consultation rates and incidence rates of intercurrent morbidity among patients with chronic disease in general practice. Br J Gen Pract 1994;44:259–62. Pubmed
50. Gurwitz JH, Col NF, Avorn J. The exclusion of the elderly and women from clinical trials in acute myocardial infarction. JAMA 1992;268:1417–22. Pubmed
51. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:M255–63. CrossRef Pubmed
52. Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-Dose Combinations Improve Medication Compliance: A Meta-Analysis. AM J Med 2007; 120(8):713-719. CrossRef Pubmed
53. Webster R, Patel A, Selak V, Billot L, Bots ML, Brown A, et al. Effectiveness of fixed dose combination medication (‘polypills’) compared with usual care in patients with cardiovascular disease or at high risk: A prospective, individual patient data meta-analysis of 3140 patients in six countries. Int J Cardiol. Netherlands; 2016; 205: 147–156. https://doi.org/10.1016/j. ijcard.2015.12.015 PMID: 26736090 CrossRef Pubmed
54. Häring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24- week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36(11):3396-404. CrossRef Pubmed
55. Chris Feudtner. Collaborative Communication in Pediatric Palliative Care: A Foundation for Problem-Solving and Decision-Making. Ped Clinics North America. October 2007; 54(5): 583-607. https://doi.org/10.1016/j. pcl.2007.07.008 CrossRef Pubmed
56. Stephen Jay Gould. Medical Narrative: The Median Isn’t the Message. Am Med Assoc J Ethics, January 2013;15(1):77-81.pp 80. CrossRef
57.Chapter Two: Things Fall Apart. In Atul Gawande. Being Mortal: Medicine and What Matters in the End. Metropolitan Books. NY, October 2014
58. S Freeman, D Critchley and L Lee. ‘Cradle to Grave’. In M Wynants (ed.). In Sickness and in Health: The future of medicine – Added value and global access. Brussels: Vrije Universiteit Brussel Press.
Copyright Information
