Case Report
September 2020, 28:1
First online: 23 October 2020
Case Report
Percutaneous Pulmonary Valve Implantation in a Bangladeshi Center: A Case Series
Prof Nurun Nahar Fatema, Congenital and Structural Interventionist, FCPS, FRCP (Edin), FACC, FSCAI, Head of Pediatric Cardiology,1 Dr Mansur Al Jufan, MD, Structural and Interventional Cardiologist,2 Prof Stephan Shubert, MD, Consultant Pediatric Cardiologist,3 Dr Mir Mahmud Hossain, FCPS, Chief Cardiac Anaesthesiologist1
2 King Faisal Specialist Hospital and Research Center, Riyadh, KSA
3 German Heart Center, Berlin
Correspondence: Prof Brig Gen Nurun Nahar Fatema, Room no 215, Lab Aid Cardiac Hospital, Road 4, Dhanmondi, Dhaka, Bangladesh.
E mail: colfatema@hotmail.com
ABSTRACT
INTRODUCTIONIncidence of congenital heart disease is 25 per thousand live birth in Bangladesh which is much higher than other countries. Tetralogy of Fallot, the commonest cyanotic heart disease (5%) and some other complex diseases with right ventricular outflow tract abnormality demand surgical correction and revision in many occasions including percutaneous intervention. As a resource constraint country, it was a difficult task to introduce percutaneous pulmonary valve implantation (PPVI) with Melody™. However, it was started on 12th December 2012 in Combined Military Hospital Dhaka, Bangladesh and cases performed till October 2019 were included in this series.
METHODS
Retrospective analysis of six cases who had PPVI with Melody™ in Combined Military Hospital, Bangladesh. Patient with dysfunctional conduit between right ventricle (RV) and pulmonary artery causing (a) Symptoms of exceptional dyspnoea of various grade (NYHA II,III, IV) (b) RVEVD >150 ml/m² ±regurgitant fraction >40% (c) RVOT peak instantaneous gradient > 30 mm Hg. (d) RV dysfunction (RVEF<40%) were accepted for the procedure and outcome were analyzed.
RESULTS
Mean age was 9.56 ± 2.96 years, weight was 28.75 ± 8.61 kg, height was 137.5 ± 17.52 cm. Mean age at surgery was 4.25 ± 2.72 years. Female were 66.66%. Aortic homograft was used in 66.66% cases. Eighteen mm Ensemble was used in four (66.66%) cases and 20 mm and 22 mm in one each. Immediate result was excellent with no residual PS in two cases and negligible residual flow acceleration across pulmonary valve in four cases. No PR seen in all except one. One patient developed Bacterial endocarditis after 3 years and was treated.
CONCLUSION
Aim of PPVI is to prolong the life expectancy of conduits which were placed surgically from right ventricle to pulmonary artery. In our case series, we found that Melody valve is functioning well without any complications like infective endocarditis or stent fracture.
KEYWORDS
PPVI, Melody™, RVOT, Outcome
INTRODUCTION
Percutaneous Pulmonary valve implantation was first introduced by Bonhoeffer et all in 20001 to treat stenosis & regurgitation in a prosthetic conduit in the right ventricular outflow tract (RVOT). Although surgical replacement of conduit is associated with low mortality and morbidity but operative risk is high in some cases after repeated sternotomies. In some cases surgery is also prohibited.2 Multiple sternotomies and conduit replacement throughout life span is also troublesome.3 Conduit are usually placed between right ventricle (RV) and Pulmonary artery (PA) in patient with Tetralogy of Fallot (TOF) with pulmonary stenosis or Pulmonary atresia (PA), Double outlet right ventricle (DORV) with pulmonary stenosis (PS), Truncus arteriosus (TA), Transposition of great arteries (TGA) with PS and ventricular Septal Defect (VSD) etc. In Ross procedure, a homograft in a pulmonary position also require valved conduit. Surgical correction of RVOT obstructions are usually performed by transannular patch or by implanting biological valves like homograft or xenograft.4 Since the introduction in 2000, thousands of cases has been performed in Europe, America, other regions and there is significant improvements in valve designs and procedures and all the capable centers are trying to incorporate this procedure in their routine practice.5-7 About 95% of the children with RVOT repair can survive up to adulthood, however all of them need one or two percutaneous of surgical reintervention.8-9 In this study we included the variety who had surgically implanted prosthetic valves and conduits which degenerates in course of time even calcified, leading to stenosis, regurgitation or both. We started PPVI in Combined Military Hospital (CMH) Bangladesh with Melody™ pulmonary valves on 25th December 2012 as first ever team of South Asia.10 As a resource constraint country it was difficult to introduce such expensive valve (3000 USD), moreover there was no health insurance system existing for civilian population. Patient party has to pay for the valve and accessories. Melody™ pulmonary valves in these cases were arranged from various charities and corporate social responsibilities (CSR) fund. Moreover pre stenting with covered CP stent or Andra bare metal stent and BIB balloons and other hardware increase the cost further beyond the limit. Aim of this study is to describe the characteristics and outcome of the cases.
METHODS
This is a retrospective analysis of PPVI cases using Melody™ valve (Medtronic Inc, Minneapolis, MN, USA) since December 2012 to October 2019, performed in Combined Military Hospital, Dhaka, Bangladesh. Patients underwent PPVI based on: dysfunctional right ventricle (RV) to pulmonary artery (PA) conduit with:
1. RV systolic pressure> 2/3 rd of systemic pressure in symptomatic patient.
2. RV systolic pressure > ¾ rth of systemic pressure in asymptomatic patient. 3. Moderate to severe Pulmonary regurgitation with symptoms ora) Moderate to severe RV dilatation
b) Moderate to severe RV dysfunction.
c) Impaired exercise capacity (< 65% predictive, NYHA class II, III, IV)
Specific criteria for using Melody™ were:
4. Diameter of conduit 16 mm – 22mm. 5. RVOT diameter not more than 22 mm 6. Coronary artery origin or course not closely related to RVOT. 7. Venous access adequate to allow 22 F sheath through femoral vein or Jugular Vein. All patients in this series underwent workup for PPVI. All the parameters were assessed from history, physical examination, surgical history and notes. ECG, CXR, Echocardiography were done. Echocardiographic assessment of RVOT conduit for peak pressure gradient across pulmonary valve (PS) was measured by CW doppler, pulmonary regurgitation (PR) by color doppler, RV dimensions (RVEDV ml/m², RVESV ml/m²) by Simpson method, RV ejection fraction (RVEF) by M-Mode, TR (CW Doppler) etc. Cardiac Magnetic resonance Imaging (CMRI) was not performed for non-availability. Diagnostic cardiac catheterization was performed for checking morphology of RVOT more accurately by balloon sizing and aortogram to exclude coronary compression during balloon occlusion of RVOT.Melody™ pulmonary Valve: We used Melody™ Valve (Medtronic Inc, Minneapolis, MN) which was approved by United States FDA in 2010 with HDE (Humanitarian device exemption). It consist of a harvested valve from a bovine jugular vein and is sutured into a platinum – iridium stent frame which is 28 mm long and 18 to 22 mm diameter. Crimped diameter is 6 mm.
Delivery System: The stent is crimped onto the Ensemble delivery system and can be expanded up to 22 mm diameter. It is a 22 French catheter with a balloon in balloon deployment design and has 18, 20, 22 mm outer diameter. The tip of the system is blue which corresponds with outflow suture of the stent. There are three proximal port, green one for guidewire, indigo for inner balloon and orange for outer balloon.
Statistical analysis: Data were collected using Excel spread sheets. Results were presented as mean ± standard deviation (SD), cases were shown individually, paired t test done where applicable.
Table 1 showed demographic profile of patients. Mean age was 9.56 years ± 2.96 years. Female (66.66%) outnumbered male (33.33%). Mean weight was 28.75 ± 8.61 kg. Height varied from 137.5 ± 17.52 cm. Age at the time of surgery was 4.25 ± 2.72 years. Four cases (66.66%) had pulmonary atresia and two had tetralogy of Fallot (33.33%). Four cases had Aortic homograft (66.66%), one had pulmonary homograft (16.67%) and one had Bovine valved pericardial conduit (116.67%). Four cases had BT shunt followed by Rastelli operation (66.66%), One had ToF repair followed by redo surgery within one year (16.67%) and one had single surgery (16.67%). Table 2 showed Echocardiographic findings of study cases. Conduit stenosis was 45 mm Hg or more in all cases and regurgitation was moderate in 2 cases & severe in 4 cases. RVEDV was > 130 ml/m² in all cases. One patient had absent LPA, and one had RPA stenosis, one had RPA and LPA stenosis along with conduit stenosis, Table 3 Showed procedural data. General anesthesia (GA) was given in all cases, Access was transfemoral for all. Two CP stents were implanted in three cases and one stent each in rest three cases. Mean fluoroscopy time was 65 ± 9.36 minutes. Melody™ with 20 mm and 22 mm ensemble was used in two cases and 18 mm in four cases. Immediate complications like device embolization, dislocation or arrhythmia were not observed in any cases. Additional procedure were Balloon angioplasty (RPA) in one case (case 2) and RPA and LPA origin balloon angioplasty in one case (case 5). Table 4 showed Immediate outcome of the patient. Residual negligible stenosis of 10 to 16 mm Hg was observed in four cases. Two cases had no gradient. No residual PR was observed in 5 cases and trivial in one case. P-value was significant.
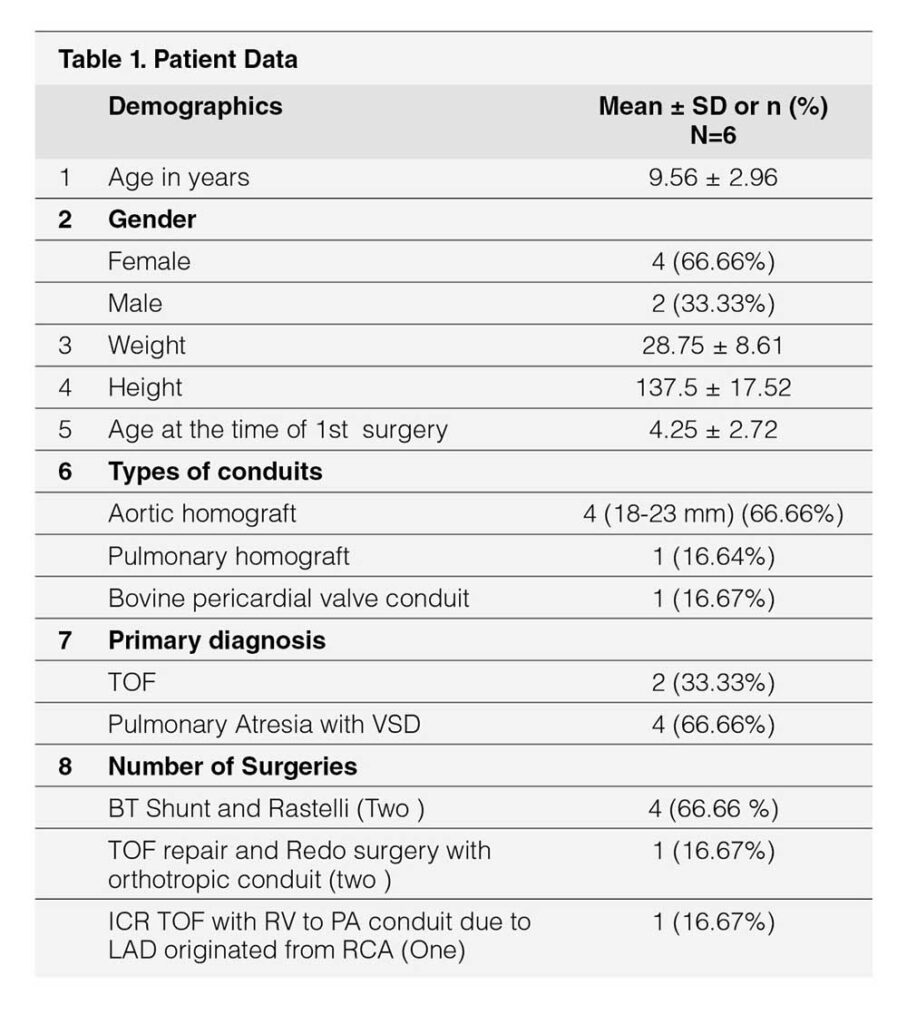
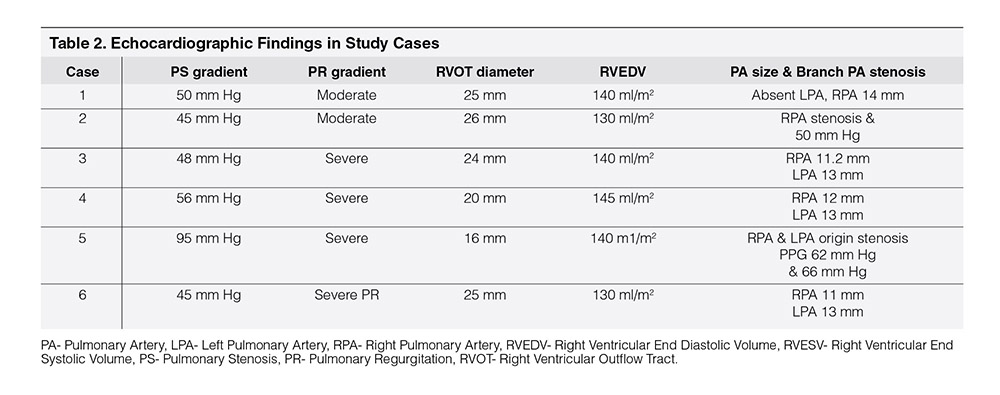
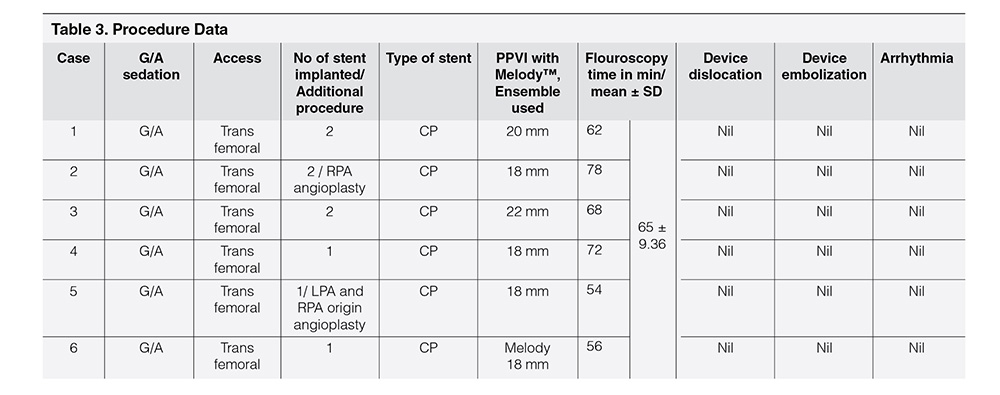
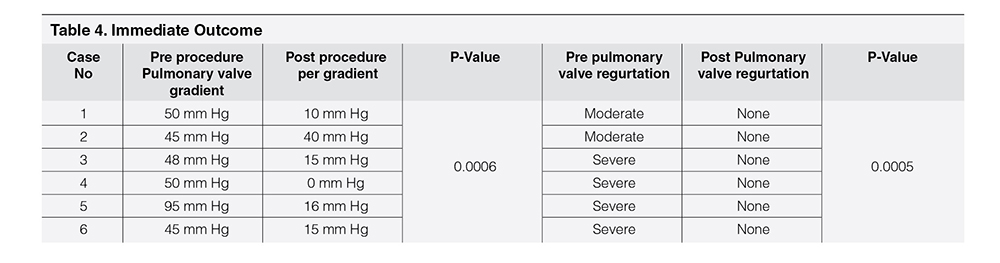
Table 5 showed follow up of cases for any PS, PR, Infective endocarditis, and stent fracture. First case completed 8 years follow up. No complication was noticed. 2nd case was lost from follow up after 3 years, she had endocarditis after three years and was treated for 6 weeks, cured, and then lost from follow up. Third and fourth cases are in follow up for four years and no complications noted, fifth and sixth cases are in follow up for 6 months and had no complications.
Figure 1 showed RV angiogram with measurements.
Figure 2 and figure 3 showed stages of Melody™ valve implantation.
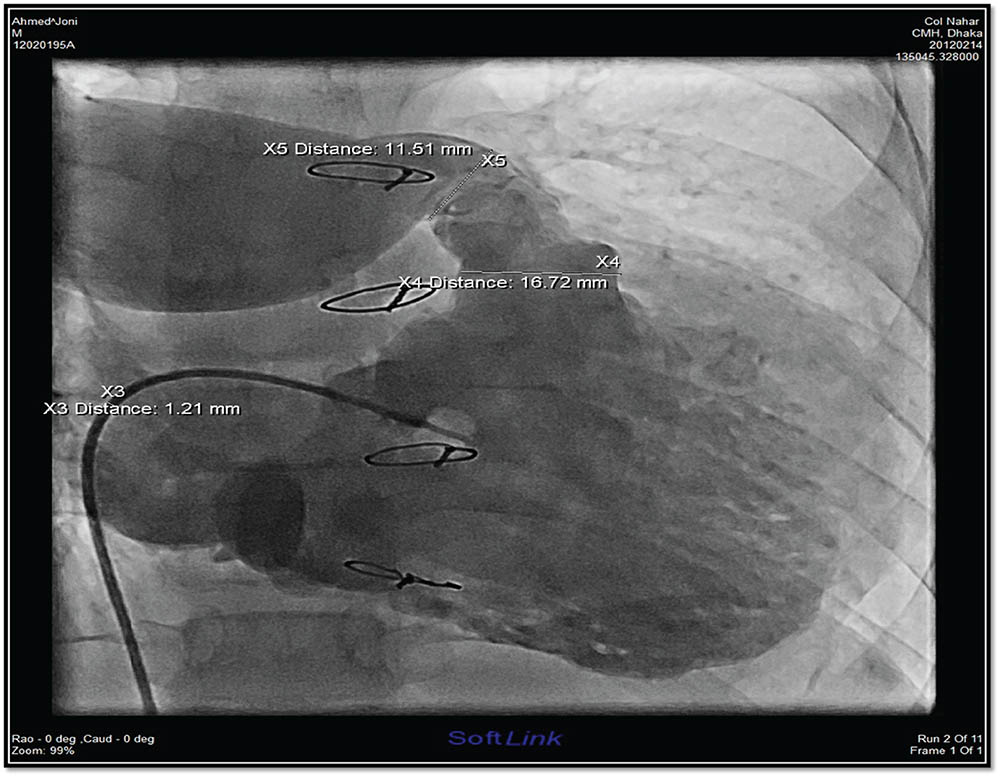
Figure 1
RV angiogram in case 1 showing absent LPA and conduit stenosis.
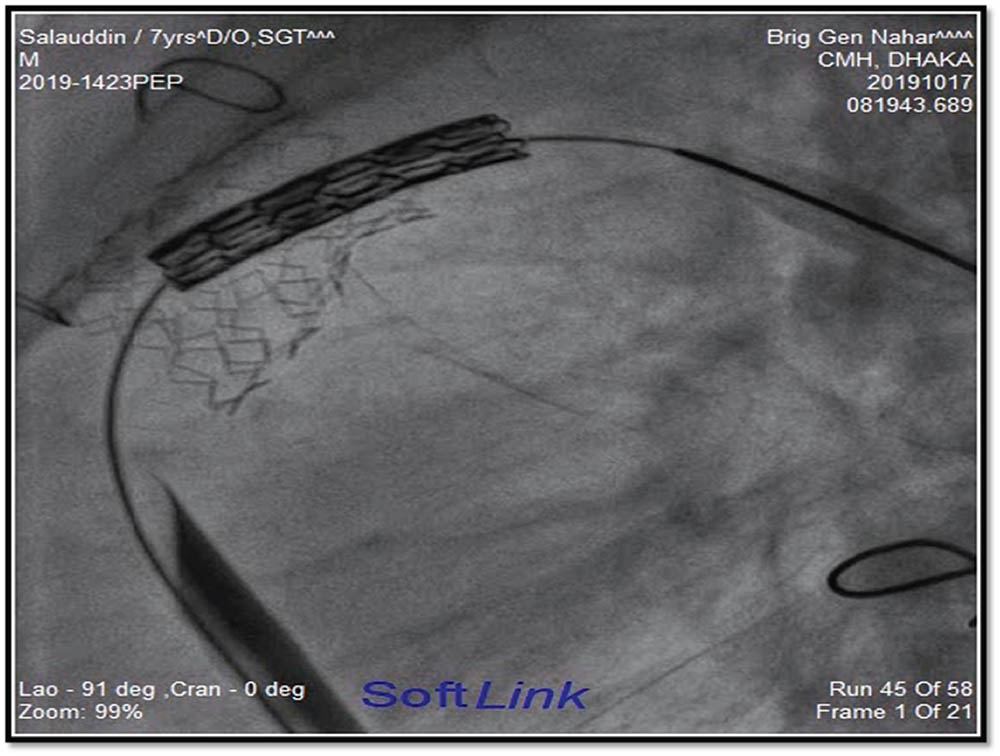
Figure 2
Melody™ valve is ready for implantation.
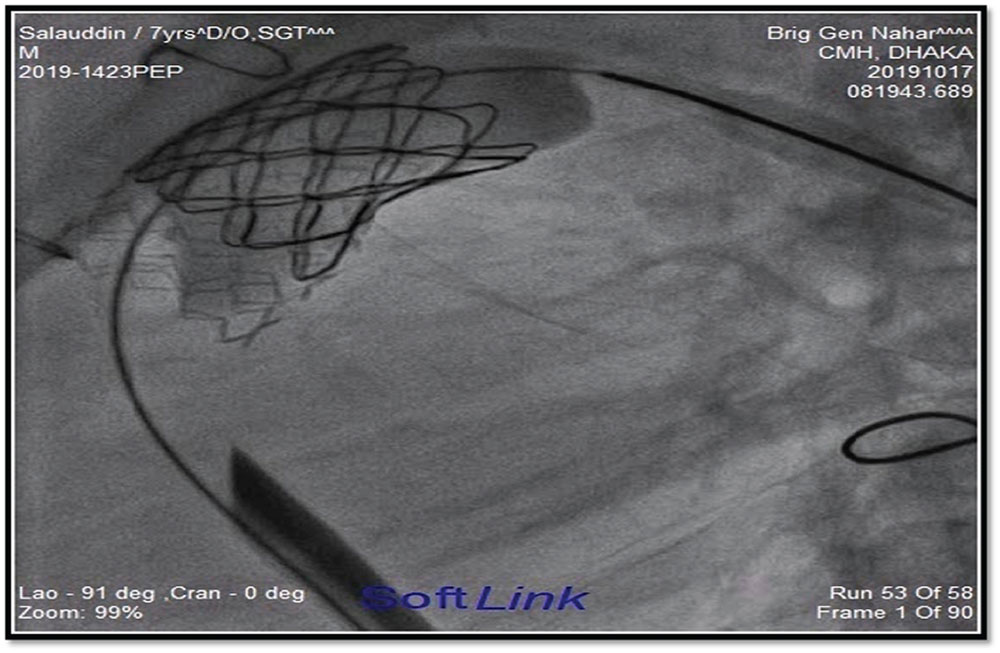
Figure 3
Melody™ valve after complete dilatation.
DISCUSSION
Approximately 20% congenital heart disease has abnormalities in right ventricular outflow tract11 eg. Tetralogy of Fallot (TOF), Pulmonary atresia, Transposition of great arteries (TGA) with pulmonary stenosis, Truncus arteriosus etc. Following surgical repair, RVOT dysfunction is very common in these patients in the form of pulmonary valve regurgitation (PR) or pulmonary valve stenosis (PS), or both.1-4 Many of them require change of conduits several times in their life span by surgical pulmonary valve replacement (SPVR) as conduit has limited life span.8-14 PPVI is an alternative treatment modality which could delay surgery by prolonging conduit life span and reducing the number of operations on the open heart. Since first implantation in 2000 by Phillip Bonhoeffer, thousands of cases were performed all over the world.[15, 16] Points in favor of doing PPVI than SPVR is (1) It delays the next surgery (2) Cost effective comparing to multiple surgery in life span of a patient (3) Outcome is good and hospital stay is less (4) In good hand procedure is very safe.17-19 We did first case of our series as first ever case in South Asia on 25th December 2012.10 In Bangladesh Pediatric Cardiology service was started in 1998. Since then patients were referred for RVOT reconstruction to surgeons. As there is no insurance support for cardiac treatment, many patients cannot manage cost of first surgery even. Moreover, many of them need multiple surgeries.[20] In outpatient department, many patients are encountered with RVOT dysfunction with multiple previous surgeries but they could not afford PPVI as valve is very expensive. So in our set up it is very difficult to enroll a patient in first place. Out of six cases in the series, valves were arranged by personal effort of cardiologist with help of charity or donation. There were many hurdles other than monetary crisis. Important one was non availability of cardiac magnetic resonance imaging (CMR) of heart. So, patients were included in the PPVI list based on detail echocardiography, CT Angiogram, and diagnostic cardiac catheterization. During catheterization, balloon sizing of the RVOT was performed to see the morphology of RVOT. Coronaries arteries were checked for any compression simultaneously. Valves were ordered after confirming the case as perfect as possible. Mean age of patient in this series was 9.56 ± 2.96 years (Table 1) which is much less than other similar studies.11 Age is comparatively less as pediatric cardiology service was started in our country only two decades ago, so patients who underwent surgery since then are still young. Mean age at the time of surgery was 4.25 ± 2.72 years, 66.66% were female in this series which does not correlates with others study.9-11 Primary disease was tetralogy of Fallot in two cases and pulmonary atresia in 4 cases (TOF variant). These correlates with other studies.20-21
Doppler Echocardiography was used to measure RV pressure from TR gradient, RVOT gradient and quantitative color flow was used for PR grading.22, 23 CMR and CT angiography techniques differ in terms of spatial, temporal resolution and radiation dose. Cardiac CT offers the advantage of high spatial resolution, fast acquisition at the expense of poor temporal resolution and ionizing radiation. CT angio can give proper idea about24 various diameters, calcification of conduits etc. A study published in European Journal of Radiology stated that CT analysis prior to percutaneous pulmonary valve implantation (PPVI) could help cardiologists to detect risk of coronary compression.25 So invasive balloon occlusion aortogram to check coronary compression in high risk cases can be avoided by excluding them from PPVI list.
Relief of RVOT obstruction and pulmonary valve frequently results in PR which initiates a cascade of events leading to RV dilatation followed by dysfunction. There is also chance of RVOT or pulmonary artery stenosis, RVOT aneurysm, TR, LV dysfunction, AR, aortic root dilatation etc. Anatomy is complex sometime and associated with RVOT aneurysm.26, 27 CMR is required to delineate RVOT and to find out various volumes and ejection fraction.
In almost all centers, RVOT dimension are assessed by MR angiogram. A study compared the assessment of RVOT dimension prior to PPVI by two types of MR, contrast enhanced magnetic resonance (CEMRA) angiography versus 3D steady – state free precession sequence (SSFP). This study showed that CMR is a suitable technique for pre procedural assessment of RVOT for PPVI. Measurement of systolic SSFP showed excellent correlation with current gold standard and all suitable for precise siring of the valve. This study verifies that the RVOT diameter depends on timing of acquisition: wider in SSFP –systole and smaller in SSFP- diastole.13, 17, 27
In all cases of this series, PS gradient was more than 45 mm Hg and two cases had moderate PR, other four had severe PR, RVEDV was more than 130 ml/m² in all cases (Table 2, Echocardiographic findings). RVOT morphology was assessed from CT angiogram and diagnostic 2D cardiac catheterization.10
Cardiopulmonary exercise test was performed in two cases (case 4, case 6) only following other guidelines.28 All our cases were performed under GA which correlates with other studies (Table 3).12 CP stent was used in all cases with an aim to prevent stent fracture and valve durability.
Result of the procedure was assessed in the Catheterization Laboratory by measuring withdrawal gradient between pulmonary artery to RV (Table 4). It was “O” in two cases and negligible gradient of 10-16 mm Hg were noticed in other four cases. Pulmonary artery angiogram showed no PR in all cases except one with trivial PR. It correlates with other studies.19-32 In follow up visits, echocardiography was done to look for (1) PS (2) PR (3) RVEDV (4) RVEF (5) TR etc. Chest X-Ray was done to look for stent fracture and electrocardiography (ECG) for arrhythmia. In follow up echocardiography RVEDV was reduced in all cases which correlates with other studies.22-25 RVOT gradient was not increased and trivial PR was noticed is one case, RVEF also improved. Most common complication seen in other study is stent fracture.33, 34 In this series, no stent fracture was noticed (Table 5). In another study stent fracture was observed in 5% cases.34 Infective Endocarditis is a potential threat for PPVI cases.35, 36 In one (Case no 2) of our case, infective endocarditis was diagnosed after 3 years of PPVI, was treated successfully but lost from follow up there after. In our experience preparation of catheterization laboratory with all accessories presuming any kind of complications which may happen, ensuring supply of all hardware for an infrequent procedure considering resource constraint setup was a challenge. Availability of patient with conduit dysfunction in the background of only two decade history of pediatric cardiac services is also a factor for less number of cases. To fulfill operational and institutional requirement, a guideline is also required for successful outcome of PPVI.37, 38
In spite of above challenges, all six cases had 100% procedural success with no significant complications.
CONCLUSIONS
Outcome of the cases revealed from this series was rewarding. Only one case suffered from infective endocarditis and was cured after treatment. No other complications were noticed in follow up. We can recommend this procedure for resource constraint set up as durable and effective one in long run.
REFERENCES
1. Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, Acar P, Le Bidois J, Sidi D, Kachaner J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet 356(9239):1403–5, 2000. CrossRef Pubmed
2. Kevin Ong, Robert Boone, Min Gao, Ron Carere, John Webb, Marla Kiess, Jasmine Grewal. Right Ventricle to Pulmonary Artery Conduit Reoperations in Patients With Tetralogy of Fallot or Pulmonary Atresia Associated With Ventricular Septal Defect. Am J Cardiol. 111(11):1638-43, 2013. CrossRef Pubmed
3. Holst KA, Dearani JA, Burkhart HM, Connolly HM, Warnes CA, Li Z, Schaff HV. Risk factors and early outcomes of multiple reoperations in adults with congenital heart disease. The Annals of Thoracic Surgery 92(1):122– 8,2011. CrossRef Pubmed
4. Ansari MM, Cardoso R, Garcia D. Percutaneous pulmonary valve implantation: present statusand evolving future. JACC 66:2246-55, 2015. CrossRef Pubmed
5. Asoh K, Walsh M, Hickey E, Nagiub M, Chaturvedi R, Lee KJ, Benson LN. Percutaneous pulmonary valve implantation within bioprosthetic valves. Eur Heart J 31(11):1404–9, 2010. CrossRef Pubmed
6. Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, Muthurangu V, Lee TY, Parenzan G, Derrick G, Cullen S, Walker F, Tsang V, Deanfield J, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation 117(15):1964–72, 2008. CrossRef Pubmed
7. McElhinney DB, Hellenbrand WE, Zahn EM, Jones TK, Cheatham JP, Lock JE, Vincent JA. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation 122(5):507–16, 2010. CrossRef Pubmed
8. Jones MI, Qureshi SA. Recent advances in transcatheter management of pulmonary regurgitation after surgical repair of tetralogy of Fallots. F1000Res.7. (pubmed) 2018.CrossRef Pubmed
9. Carminiti M, Pluchinotta FR , Piazza L. Echocardiographic assessment after surgical repair of tetralogy of Fallot. Front Pediatr 3:3,2015.CrossRef Pubmed
10. NN Fatema, MR Hossain. Percutaneous Pulmonary Valve Implantation (PPVI) with Melody®: First Ever Case Report in South Asia. BCPS Journal 32:102-106,2014. CrossRef
11. Anoosh Esmaeili, Markus Khalil, Kachina Behnke Hall, Maria Belen Gonzalez y Gonzalez, GuterKurst, Stephen Fichtlscheler et al. Percutaneous pulmonary valve implantation (PPVI) in non-obstructive right ventricular out flow tract: Limitations and midterm outcomes. Translational Pediatrics 8(2):107,2019. CrossRef Pubmed
12. Liyu Ran,* Wuwan Wang,* Francesco Secchi, Yajie Xiang, Wenhai Shi, and Wei Huang. Percutaneous pulmonary valve implantation in patients with right ventricular outflow tract dysfunction: a systematic review and metaanalysis. Ther Adv Chronic Dis 2019; Published online 2019 Jun 14. doi: 10.1177/2040622319857635 CrossRef Pubmed
13. Khambadkone S, Coats L, Taylor A, Boudjemline Y, Derrick G, Tsang V, Cooper J, Muthurangu V, Hegde SR, Razavi RS, Pellerin D, Deanfield J, Bonhoeffer P. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation 112(8):1189–97, 2005. CrossRef Pubmed
14. O’Byrne ML, Glatz AC, Mercer-Rosa L, et al. Trends in pulmonary valve replacement in children and adults with tetralogy of Fallot. Am J Cardiol 115:118–124, 2015. CrossRef Pubmed
15. Borik S, Crean A, Horlick E, et al. Percutaneous pulmonary valve implantation: 5 years of follow-up does age influence outcomes? Circ Cardiovasc Interv 8: e001745. 2015. CrossRef Pubmed
16. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation 131: 1960–1970, 2015. CrossRef Pubmed
17. Yuan S-M, Mishaly D, Shinfeld A, et al. Right ventricular outflow tract reconstruction: valved conduit of choice and clinical outcomes. J Cardiovasc Med 9: 327–337, 2008. CrossRef Pubmed
18. Kanter KR, Budde JM, Parks WJ, et al. One hundred pulmonary valve replacements in children after relief of right ventricular outflow tract obstruction. Ann ThoracSurg 73: 1801–1806, 2002.CrossRef Pubmed
19. Hallbergson A, Gauvreau K, Powell AJ, et al. Right ventricular remodeling after pulmonary valve replacement: early gains, late losses. Ann Thorac Surg 99: 660–666, 2015. CrossRef Pubmed
20. Therrien J, Provost Y, Merchant N, et al. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol 95: 779–782, 2005. CrossRef Pubmed
21. Eicken A, Ewert P, Hager A, et al. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. Eur Heart J 32: 1260–1265, 2011. CrossRef Pubmed
22. Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by doppler ultrasound in patients with tricuspid regurgitation. Circulation 70:657-662, 1984. CrossRef Pubmed
23. Dragulescu A, Friedberg MK, Grosse Wortmann L, Redington A, Mertens L. Effect of chronic right ventricular interaction in patients after tetralogy of fallot repair. J Am Soc Echocardiography 27;896-902, 2014. CrossRef Pubmed
24. Michellatezza, Maarten Witsenburg, Koen Nieman, Pieter C Van De Woestjine, Riccardo P.J Budde. Cardiac CT to assess the risk of coronary compression in patient evaluated for percutaneous pulmonary valve implantation. European Journal of cardiology 11;018, 2018. CrossRef Pubmed
25. Morray BH, McElhinney DB, Cheatham JP. Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. Circ Cardiovasc Interv 6:535- 42,2013. CrossRef Pubmed
26. Geva T. Repaired tetralogy of Fallot: The roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson 13:9, 2011. CrossRef Pubmed
27. Sebastian Evel, Sebastian Gottschling, Christian Lucke. 3D assessment of RVOT dimension prior percutaneous pulmonary valve implantation comparison of contrast enhanced magnetic resource angiography versus 3D steady state free precession sequence. The International Journal of Cardiovascular Imaging. 35:1453-1463, 2019. CrossRef
28. Batra AS, McElhinney DB, Wang W, et al. Cardiopulmonary exercise function among patients undergoing transcatheter pulmonary valve implantation in the US Melody valve investigational trial. Am Heart J 163: 280–287, 2012. CrossRef Pubmed
29. P Lurz, L Coats, S Khambadkone. Percutaneous pulmonary valve implantation: Impact of evolving technology and learning curve on clinical outcome. Circulation 117:1964-1972,2008. CrossRef Pubmed
30. Khambadkone S, Coats L,Taylor A, Boudjemline Y, Tsang V, Cooper J, Muthurangu V, Hegdeha SR, Razavi RS, et al. Percutaneous pulmonary valve implantations in humans; results in 59 consecutive patients. Circulation 112:1189-1197, 2005. CrossRef Pubmed
31. Fiszer R, Dryzek P, Szkutnik M, et al. Immediate and long-term outcomes of percutaneous transcatheter pulmonary valve implantation. Cardiol J 24: 606–611, 2017. CrossRef Pubmed
32. Butera G, Milanesi O, Spadoni I, et al. Melody transcatheter pulmonary valve implantation. Results from the Registry of the Italian Society of Pediatric Cardiology (SICP). Cardiol Young 22:S26, 2012. CrossRef Pubmed
33. Fraisse A, Aldebert P, Malekzadeh-Milani S. Melody® transcatheter pulmonary valve implantation: results from a French registry. Arch Cardiovasc Dis 107:607–614, 2014 CrossRef Pubmed
34. McElhinney DB, Cheatham JP, Jones TK, et al. Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation: patient-related and procedural risk factors in the US melody valve trial. Circ Cardiovasc Interv 4:602–614, 2011. CrossRef Pubmed
35. Nordmeyer J, Lurz P, Khambadkone S, et al. Pre-stenting with a bare metal stent before percutaneous pulmonary valve implantation: acute and 1-year outcomes. Heart 97:118–123, 2011. CrossRef Pubmed
36. Buber J, Bergersen L, Lock JE, et al. Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: A single-center experience. Circ Cardiovasc Interv 6:301–31, 2013. CrossRef Pubmed
37. Villafañe J, Baker GH, Austin EH, et al. Melody® pulmonary valve bacterial endocarditis: experience in four pediatric patients and a review of the literature. Catheter Cardiovasc Interv 84:212–218, 2014. CrossRef Pubmed
38. Hijazi ZM, Ruiz CE, Zahn. Operator and institutional requirements for transcatheter valve repair and replacement, part III: pulmonic valve. J Am Coll Cardiol 65:2556–2563, 2015.CrossRef Pubmed
Copyright Information
