Case Report
August 2024, 33:2
First online: 30 August 2024
Case Report
Rare and Susceptible: Hypermucoviscous Capsular 1 (K1) Serotype Klebsiella Pneumoniae Infective Endocarditis in a Patient with HIV-AIDS and Chronic Lymphocytic Leukemia
Pilapil, John Christopher A.;1 Chiu, Harold Henrison C.;2 Gabunada, Ron Rafael W.;3 Pajares, Corilyn V.;4 Alejandria, Marissa M.;3 Dela Tonga, Angelo D.5
3 Division of Infectious Diseases, Department of Medicine, University of the Philippines – Philippine General Hospital
4 Division of Hematology, Department of Medicine, University of the Philippines – Philippine General Hospital
5 Institute of Molecular Biology and Biotechnology, National Institutes of Health, University of the Philippines Manila, Manila, Philippines
Main Author’s Name: John Christopher A. Pilapil
Main Author’s Contact Details:
Email Address: jcapilapil@gmail.com.
Cellular Number: +639178555227
ABSTRACT
Hypermucoviscous (hv) Klebsiella pneumoniae strains are known to cause community-acquired infections that are serious, metastatic, and lethal. Patients with uncontrolled diabetes mellitus and people of Asian descent are predisposed to more severe disease. Infective endocarditis is an extremely rare complication of these infections. A 41-year-old Filipino male with HIV-AIDS and chronic lymphocytic leukemia presented with fever and symptoms of acute heart failure. He had hypotension, engorged neck veins, bibasal crackles, and a holosystolic murmur at the cardiac apex. Echocardiography showed an oscillating mass on the anterior mitral valve. Blood cultures grew hypermucoviscous K. pneumonia with positive string test and confirmed via multilocus sequence virulence typing. He improved with six weeks of intravenous antibiotics.This is the first case of (1) hv phenotype K. pneumoniae infective endocarditis, without other infectious foci, in a non-diabetic man with HIV-AIDS and CLL; and (2) an hv K. pneumoniae endocarditis patient with shock to have survived.
INTRODUCTION
Hypermucoviscous (hv) Klebsiella pneumoniae is a hypervirulent, community-acquired strain of K. pneumoniae known to cause serious, lethal, and metastatic infections. They are notable for their predisposition towards patients with uncontrolled diabetes mellitus and in the healthy population, patients who are of Asian descent. Various reports of the disease entity caused by these strains show it to cause multifocal and disseminated infections presenting as liver abscesses, conjunctivitis, endophthalmitis, osteomyelitis and epidural abscess, pneumonia, and septic pulmonary embolism.1-4 Infective endocarditis is an extremely rare complication of hv K. pneumoniae disease. Here, we present the first case of hv phenotype K. pneumoniae infective endocarditis in the absence of a well-defined infectious foci in a non-diabetic, middle-aged man diagnosed to have HIV-AIDS and chronic lymphocytic leukemia. In this report, we see the irony that despite the virulent nature of the pathogen causing rare infective endocarditis with cardiogenic and septic shock, it remained susceptible to a wide range of antibiotics. This is also the first report of a patient with hv K. pneumoniae endocarditis in shock to have survived.
CASE PRESENTATION
A 41-year-old Filipino male presented to our institution with a chief complaint of easy fatigability. He was diagnosed with human immunodeficiency virus (HIV) infection and acquired immunodeficiency syndrome (AIDS) since 2009. That same year, he was treated for clinically diagnosed pulmonary tuberculosis. The patient had been maintained with good compliance on lopinavir, ritonavir, tenofovir, and lamivudine, with no prophylactic antibiotics being taken. His latest CD4 count was 350 cells per microliter and he had a viral load of 48 copies per milliliter, performed 2 months before admission. Prior to admission, he had pending investigation for pancytopenia that had been present for only a few months. He was previously admitted for a single day for supportive blood transfusion for anemia and thrombocytopenia. Bone marrow aspiration and flow cytometry were done during that admission; the results of which (CD11c: moderately bright; CD19, CD20, and CD23: moderately bright; and CD5: moderately bright) revealed the presence of chronic lymphocytic leukemia (CLL), Rai high risk and Binet C. Contemplated plans at that time were to do chemotherapy. However, 3 days following discharge, the patient complained of weakness and malaise, with accompanying fever, progressive fatigue, non-productive cough, shortness of breath, orthopnea, and paroxysmal nocturnal dyspnea. Persistence of his symptoms prompted consult at our emergency room.
Pertinent physical examination findings included the following: the patient appeared cachetic with a body mass index of 17.4 and weak-looking. Patient was hypotensive (blood pressure 70/40 mmHg), tachycardic (137 beats per minute), tachypenic (25 breaths/minute), and had an axillary temperature of 38.1 °C (100.6 °F). His neck veins were engorged and had a jugular venous pressure of 6 mm H2O. Oral examination revealed multiple dental caries and thrush on the posteromedial and lateral third of his tongue. Chest exam showed fine bibasal crackles. Cardiac physical examination revealed a non-displaced apex beat, no heaves or thrills, and a grade 3/6 holosystolic murmur that was heard best over the cardiac apex, with radiation to the axilla, base, and left parasternal border; it was louder on expiration and softer on inspiration. The patient had a non-palpable liver edge, but an obliterated Traube’s space. His palpebral conjunctivae were pale and the patient’s extremities were cool, with no edema, but with observed ecchymoses and petechiae on his lower extremities. He had small, non-tender, maculopapular nodules on his palms and soles – consistent with Janeway’s lesions. Neurologic exam was unremarkable.
INVESTIGATIONS
Focused 2D-echocardiography showed a 0.4 x 0.5 cm oscillating mass on the atrial side of the anterior mitral valve (Figure 1), with an anteriorly directed jet of severe mitral regurgitation. The posterior mitral valve was stiff and prolapsed into the left atrium during systole. The left ventricle had normal cavity size and wall thickness, with adequate contractility, and an ejection fraction was 64% via biplane disk method. The left atrium was not dilated. The patient’s hemoglobin and platelets were low at 77 g/L and 7 x 109/L, respectively. White blood cell count was at 26.0 x 109/L, comprised of 99% lymphyocytes, 1% monocytes, and an absolute neutrophil count of 0. Creatinine was elevated at 126 umol/L with an estimated glomerular filtration rate of 60.6 mL/min/1.73 m2. The rest of his serum chemistry, urinalysis, prothrombin time with international normalized ratio, and partial thromboplastin time were within normal values. Three sets of blood cultures were extracted.
Gram staining of all sets of blood samples sent were with visualized gram-negative bacilli. Final results and speciation revealed Klebsiella pneumoniae. Sensitivity testing showed that the isolated species were resistant only to ampicillin and susceptible to all other antibiotics.
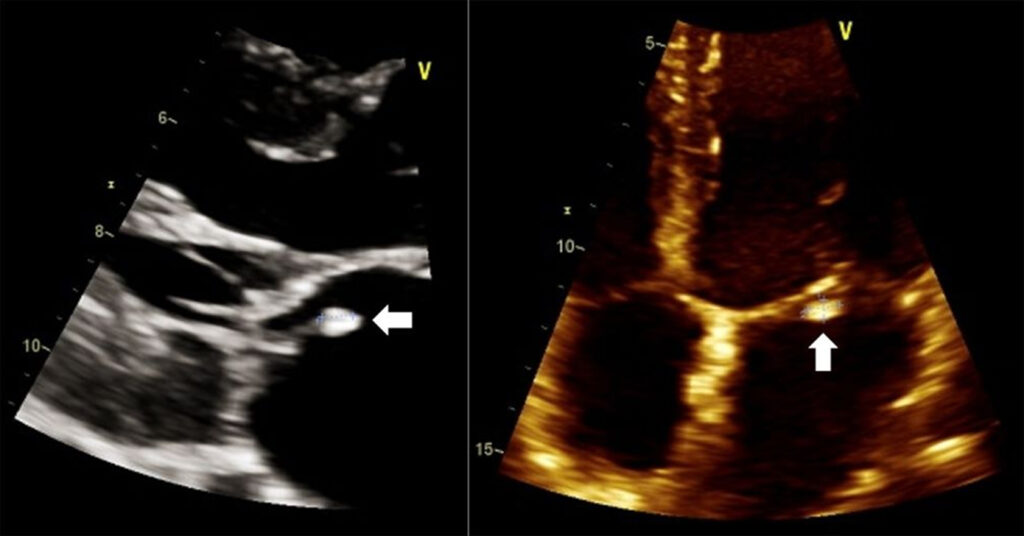
Figure 1: Transthoracic echocardiogram in parasternal long axis (left) and apical four-chamber view (right) showing a vegetation (white arrow) on the anterior mitral valve.
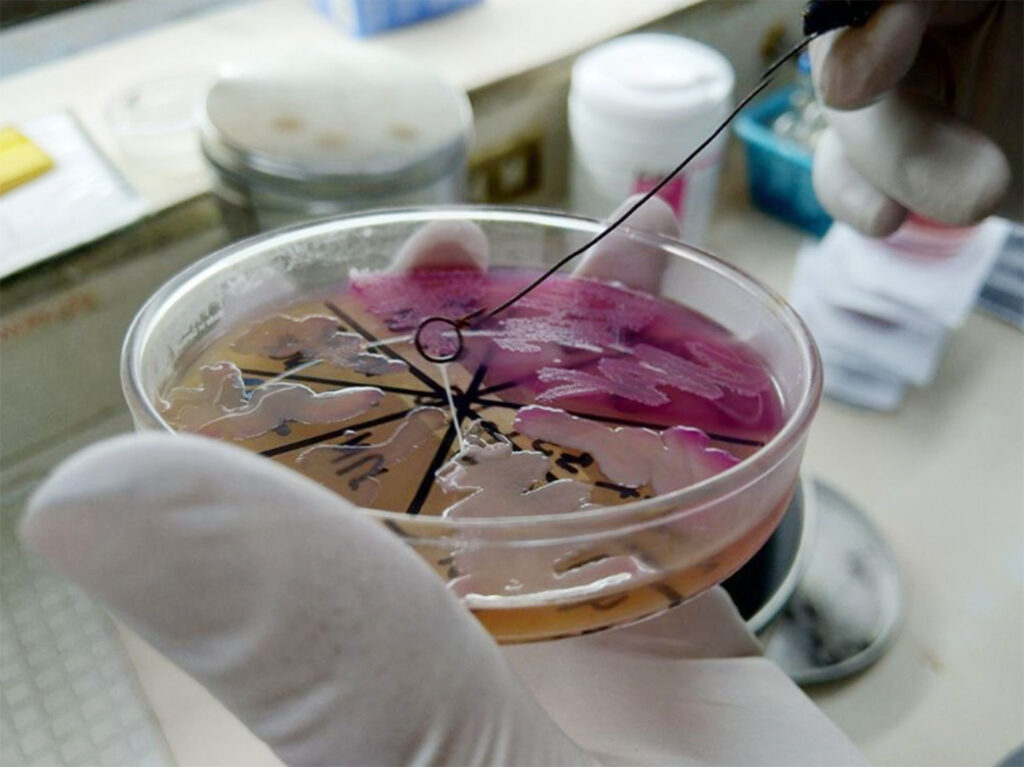
With suspicion of hypermucoviscous/hypervirulent K. pneumoniae as the etiologic agent, further testing was performed. A positive string test (Figure 2) was demonstrated by using a loop to stretch the bacterial colony on an agar plate with the subsequent formation of viscous strings well beyond 5 mm. Samples were sent to the University of the Philippines National Institutes for Health for detection of virulence genes and capsular type using multilocus sequence typing. All specimens were identical with K1 serotype and contain the iutA (aerobactin receptor), rmpA and rmpA2 (regulator of hypermucoid phenotype A) genes. They were found to be closely related to two other samples: one reported in isolates from abdominal infection and other invasive infections5 and another previously reported in hypervirulent K. pneumoniae necrotizing fasciitis and metastatic endophthalmitis from our institution as well.6 Isolates were deemed to be community-acquired, given susceptibility to most antibiotics in contrast to the institution’s antibiogram of typically multidrug-resistant organisms, including Klebsiella species.
A holoabdominal ultrasound and subsequently, an abdominal computed tomography scan with intravenous contrast were done revealing no metastatic abscesses. Ophthalmology and medical retina services noted cataracts in both eyes and suspicious HIV retinopathy on the right. There were no signs of endophthalmitis or other ocular findings consistent with any sequelae of infective endocarditis. The dentistry service seeing to the patient assessed him to have mild gingivitis and multiple dental caries.
TREATMENT
At the emergency room, the patient was started on norepinephrine (0.7 mcg/kg/minute) and dobutamine (10 mcg/kg/min). Following the extraction of initial blood cultures, he was also started on piperacillin-tazobactam (4.5 g intravenous every 6 hours), amikacin (750 mg intravenous every 24 hours), and vancomycin (1 g intravenous every 12 hours) for the treatment of septic shock in a neutropenic patient and infective endocarditis. Fluconazole was given for oral thrush and trimethoprim-sulfamethoxazole at prophylactic dose for Pneumocystis carinii infection. Supportive transfusion for the cytopenias was done. The patient was referred to the cardiovascular surgery service for possible standby emergent mitral valve surgery, with their disposition being to continue and maximize medical management. Over the next days, the patient’s condition continued to improve and he was successfully weaned from vasopressor and inotropic support.
Upon retrieval of the blood culture and sensitivity results, antibiotics were shifted to ceftriaxone (2 g intravenous every 24 hours) which the isolates were sensitive to, with plans of completing 6 weeks total of intravenous therapy. Fluconazole was eventually shifted to nystatin and cotrimoxazole was continued. Hematology service indicated plans of eventual chemotherapy for the patient’s CLL. Dentistry service advised him on oral hygiene and tooth extractions (#15, 16, and 46) were carried out on hospital day 19 of admission. They scheduled him for future outpatient procedures.
OUTCOME AND FOLLOW-UP
The patient completed a total of 6 weeks of ceftriaxone (2 g intravenous every 24 hours) for K. pneumoniae infective endocarditis and was discharged with resolution of symptoms and markedly improved heart failure functional capacity. He was sent home on carvedilol, tenofovir + lamivudine, lopinavir + ritonavir, and cotrimoxazole as maintenance medications. The patient is currently undergoing chemotherapy for the CLL with chlorambucil and rituximab.
DISCUSSION
K. pneumoniae are Gram-negative bacteria characterized to be rod-shaped, encapsulated, non-motile, facultatively anaerobic, and lactose-fermenting. The species is a known cause of pneumonia, urinary tract infection, intraabdominal, skin and soft tissue infections, and bacteremia. Among the important virulence factors of the bacteria is that of acquiring new genetic material. Two pathotypes have thus emerged, termed as classical K. pneumoniae (cKp) and hypermucoviscous or hypervirulent K. pneumoniae (hvKp).8-9
Over the past three decades, hvKp has emerged to be the causative agent of a multitude of infections – syndromes of which tend virulent, metastatic, and highly morbid, if not lethal. Most hvKp infections have quite interestingly and inordinately occurred in people of Asian descent or those living within the geographic restrictions of the Asia-Pacific Rim.6-19 However, there have been increasing cases being reported from North America, South America, the Carribean, Europe, the Middle East, Australia, Africa, and South Africa as well.8-9
A distinct array of clinical and bacterial phenotypic characteristics further sets apart hvKp from cKp. First, hv Klebsiella is able to cause serious infection in otherwise healthy and ambulatory hosts, often in a community-acquired setting as previously mentioned.20 It is worthy and important to note that diabetes mellitus is considered a risk factor – but not a prerequisite – to the development of more morbid and lethal disease.6 Secondly, hv phenotypes display the ability to produce metastatic infections. While cKp infections usually involve a single site, hvKp infections are typically multiple. Although metastatic infection can be a common sequela for Gram-positive agents (e.g. Staphylococus aureus and Streptococcus species), it is uncommonly seen in Gram-negative bacilli (classical K. pneumoniae, Proteus species, and Escherichia coli, among others) infections in the absence of an immunocompromising host condition.7-8, 11-18, 21 Third, hvKp infectious syndromes are unusual in that they involve sites apart from the lungs and urinary tract, which are typical targets for cKp. Syndromes are inclusive of, but not limited to, endophthalmitis, meningitis, brain abscess, necrotizing fasciitis, splenic abscess, epidural abscess. Fourth, hv K. pneumoniae colonies grown on an agar plate appear hypermucoviscous, as their name aptly describes. This can be semi-quantitatively identified using the string test, in which a colony is lifted via an inoculation loop or needle. The string test is positive when a viscous string longer than 5 mm is produced after a colony is stretched.8-9 Finally, as opposed to their classical counterparts that are typically hospital-acquired and carbapenem-resistant Enterobacteriaceae (CRE), these hv Klebsiella isolates were community-acquired and although glaringly invasive, were also essentially susceptible to majority of antibiotics.6, 8-9
Gram-negative infective endocarditis occurs in only around 5% of all cases of endocarditis with Klebsiella species causing around only 1.2% of native valve endocarditis and up to 4.1% of prosthetic valve endocarditis.22-23 This low rate of infective endocarditis has previously been postulated to be due to the poor adherence of Klebsiella species to cardiac valves as compared against Gram-positive and other Gram-negative species.24 The aortic valve is most commonly affected, followed by the mitral valve.1
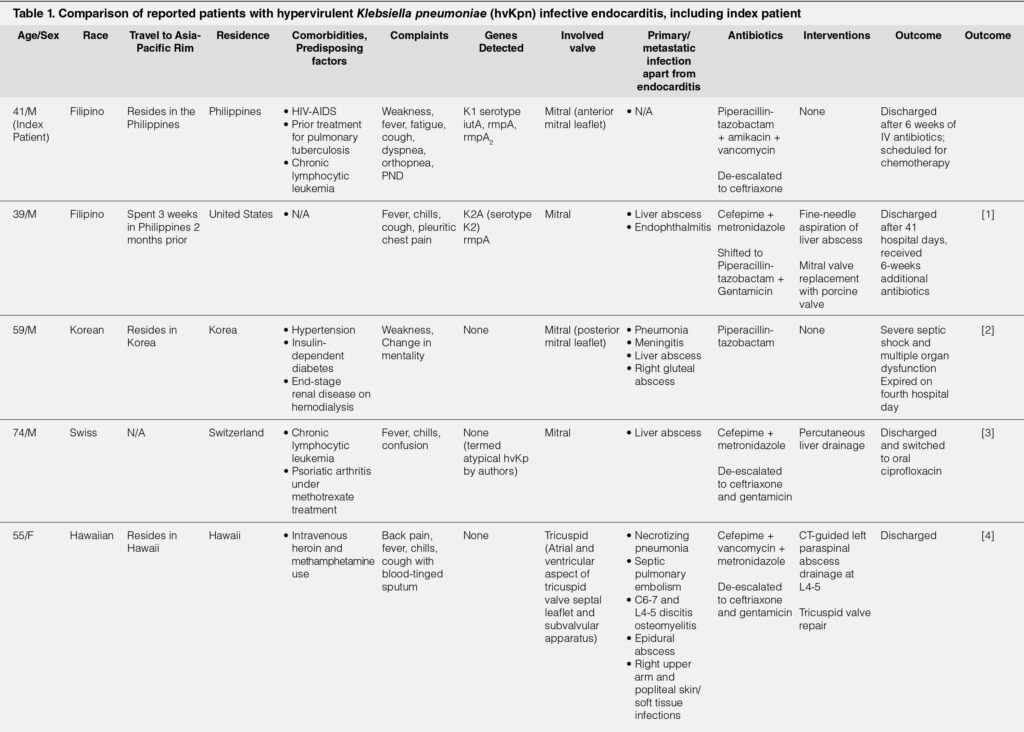
Despite the aggressiveness and virulence of hvKp, endocarditis is considered as a highly rare presentation of hvKp.1-4, 7-8 However, there are only a limited number of cases reported in literature showing that these infections have occurred mostly in people of Asian descent and/or either recent travel to or residence in the Asia-Pacific Rim. Majority of the patients also had other comorbid conditions or predisposing factors such as intravenous drug use or immunosuppressant use. Fever was a common presenting symptom in most. Common to all cases were the presence of other foci of infection – whether primary or metastatic – apart from endocarditis. Quite notably, acute heart failure was not present in any of the reported patients, nor was infective endocarditis the primary consideration or deemed infectious source upon admission. Three of the four patients survived to discharge, with one expiring after four hospital days. The only mortality was likewise the only patient who went into shock.1-4 Table 1 briefly summarizes the reported cases of infective endocarditis attributed to hvKp.
Although lymphoproliferative disorders are prevalent in HIV positive patients, only few reports describe co-existing HIV and CLL, the incidence of which is said to be 2.1 per 100,000 person-years. As their combined presence is rare, reports on their sequelae and how they predispose their hosts to other conditions and infections are scarce.25-28 Available literature suggests that for HIV patients, associated high risk activities such as intravenous drug use rather than HIV itself predisposes to endocarditis infections.29 On the other hand, infective endocarditis has not been commonly documented in patients with CLL – with the only report complicated by further immunosuppression with psoriatic arthritis undergoing methotrexate treatment.3 Most cases show endocarditis in patients with acute and not chronic leukemia. It is possible that the co-existence of HIV-AIDS and chemotherapy-naive CLL in this patient could have ultimately paved the way for such a unique clinical manifestation.
We find our patient much different in terms of presentation from all other hvKp infective endocarditis reports due to the following reasons: (1) the presence of both HIV-AIDS and chemotherapy-naïve CLL, (2) acute heart failure on admission, with the primary consideration of infective endocarditis at the onset, (3) the absence of other foci of infection, (4) survival to discharge, even after having presented in shock. In summary, we see a rare infection that is essentially – and thankfully – susceptible to antibiotics in a patient with a rare combination of diseases who was likely made susceptible to hvKp by their synergistic presence.
LEARNING POINTS/TAKE HOME MESSAGES
In summary, we highlight that
- Hypermucoviscous Klebsiella infections are set apart from their classical counterparts by often being community-acquired, metastatic and highly virulent, but susceptible to most antibiotic agents.
- Even with its already unique and odd manifestations, infective endocarditis is an extremely rare presentation of hvKp.
- In this report, we see a rare infection that was essentially susceptible to antibiotics in a patient with a rare combination of HIV and CLL who was likely made susceptible to hvKp by their synergistic presence.
- The combination of rare and susceptible in this patient likely led to a truly unique clinical manifestation but thankfully, a good outcome as well.
REFERENCES
1. Rivero A, Gomez E, Alland D, Huang DB, Chiang T. K2 serotype Klebsiella pneumoniae causing a liver abscess associated with infective endocarditis. J Clin Microbiol. 2010;48(2):639-641. CrossRef Pubmed
2. Hwang J, Her C, Kim Y. Endocarditis caused by community-acquired Klebsiella pneumoniae infection. Korean J Crit Care Med. 2013;28(1):41-45. CrossRef
3. Flury BB, Dona V, Buetti N, Furrer H, Endimiani A. First two cases of severe multifocal infections caused by Klebsiella pneumoniae in Switzerland: characterization of an atypical non-K1/K2-serotype strain causing liver abscess and endocarditis. Journal of Global Antimicrobial Resistance. 2017;10:165-170. CrossRef Pubmed
4. Riangwiwat T, Dworkin J. Tricuspid Valve Infective Endocarditis Due to Klebsiella pneumoniae in Intravenous Drug User. Hawaii J Med Public Health. 2019;78(3):98-102. Pubmed
5. Lee CR, Lee JH, Park KS, Jeon JH, Kim YB, Cha CJ, et al. Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: Epidemiology, hypervirulence-associated determinants, and resistance mechanisms. Front Cell Infect Microbiol. 2017;7. CrossRef Pubmed
6. Chiu HHC, Francisco CN, Bruno R, Jorge M, Salvaña EM. Hypermucoviscous capsular 1 (K1) serotype Klebsiella pneumoniae necrotising fasciitis and metastatic endophthalmitis. BMJ Case Rep. 2018;11:1–3. CrossRef Pubmed
7. Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi:10.1128/CMR.11.4.589. CrossRef Pubmed
8. Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107-118. doi:10.4161/viru.22718 CrossRef Pubmed
9. Russo T, Marr C. Hypervirulent Klebsiella pneumoniae. Clinical Microbiology. 2019;32(3)e00001-19. DOI: 10.1128/CMR.00001-19 CrossRef Pubmed
10. Wang JL, Chen KY, Fang CT, Hsueh PR, Yang PC, Chang SC. Changing bacteriology of adult community-acquired lung abscess in Taiwan: Klebsiella pneumoniae versus anaerobes. Clin Infect Dis. 2005;40(7):915-922. doi:10.1086/428574 CrossRef Pubmed
11. Chung DR, Lee SS, Lee HR, et al. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect. 2007;54(6):578-583. doi:10.1016/j.jinf.2006.11.008 CrossRef Pubmed
12. Tsai FC, Huang YT, Chang LY, Wang JT. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14(10):1592-1600. doi:10.3201/eid1410.071254 CrossRef Pubmed
13. Lin YT, Jeng YY, Chen TL, Fung CP. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: clinical and microbiological characteristics in Taiwan, 2001-2008. BMC Infect Dis. 2010;10:307. Published 2010 Oct 25. doi:10.1186/1471-2334-10-307 CrossRef Pubmed
14. Chang WN, Huang CR, Lu CH, Chien CC. Adult Klebsiella pneumoniae meningitis in Taiwan: an overview. Acta Neurol Taiwan. 2012;21(2):87-96. Pubmed
15. Ng D, Frazee B. Necrotizing fasciitis caused by hypermucoviscous Klebsiella pneumoniae in a Filipino female in North America. West J Emerg Med. 2015;16:165–8. CrossRef Pubmed
16. Wong CH, Kurup A, Wang YS, et al. Four cases of necrotizing fasciitis caused by Klebsiella species. Eur J Clin Microbiol Infect Dis. 2004;23:403–7. CrossRef Pubmed
17. Seo R, Kudo D, Gu Y, et al. Invasive liver abscess syndrome caused by Klebsiella pneumoniae with definite K2 serotyping in Japan: a case report. Surg Case Rep. 2016;2:1–5. CrossRef Pubmed
18. Namikawa H, Yamada K, Fujimoto H, et al. Two unusual cases of successful treatment of hypermucoviscous Klebsiella pneumoniae invasive syndrome. BMC Infect Dis. 2016;16:1–6. CrossRef Pubmed
19. Sandoval MA, Roxas MCA, Salamat M, Pauig J, Tabu I, Dela Tonga A. 3-in-1: bilateral subcutaneous leg abscesses from Klebsiella pneumoniae. BMJ Case Rep. 2020;13:e235926. doi:10.1136/bcr-2020-235926. CrossRef Pubmed
20. Pomakova DK, Hsiao CB, Beanan JM, et al. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumonia: an emerging and under-recognized pathogenic variant. Eur J Clin Microbiol Infect Dis. 2012;31(6):981-989. doi:10.1007/s10096-011-1396-6 CrossRef Pubmed
21. Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med. 1991;151(8):1557-1559. Pubmed
22. Anderson MJ, Janoff EN. Klebsiella endocarditis: report of two cases and review. Clin Infect Dis. 1998;26(2):468-474. doi:10.1086/516330 CrossRef Pubmed
23. Wu H, Li D, Zhou H, Sun Y, Guo L, Shen D. Bacteremia and other body site infection caused by hypervirulent and classic Klebsiella pneumoniae. Microb Pathog. 2017;104:254-262. doi:10.1016/j.micpath.2017.01.049 CrossRef Pubmed
24. Gould K, Ramirez-Ronda CH, Holmes RK, Sanford JP. Adherence of bacteria to heart valves in vitro. J Clin Invest. 1975;56(6):1364-1370. doi:10.1172/JCI108216 CrossRef Pubmed
25. Shohara R, Dy I, Oshikanlu Z, Savage D, Phillips A. Chronic Lymphocytic Leukemia in a Man with Human Immunodeficiency Virus: a Case Report. Blood. 2009;114(22):4400. doi: https://doi.org/10.1182/blood.V114.22.4400.4400 CrossRef
26. Shimada N, Yuji K, Ohno N, et al. Treatment of chronic lymphocytic leukemia with bendamustine in an HIV-infected patient on antiretroviral therapy: a case report and review of the literature. Clin Case Rep. 2015;3(6):453-460. doi:10.1002/ccr3.244 CrossRef Pubmed
27. England J, Leitch H. Chronic lymphocytic leukemia in a patient with wellcontrolled HIV infection: successful treatment with ibrutinib. Leukemia & Lymphoma, 2018; 59(3):752-754. doi: 10.1080/10428194.2017.1349904 CrossRef Pubmed
28. Gibson TM, Morton LM, Shiels MS, et al. Risk of non-Hodgkin lymphoma subtypes in HIV-infected people during the HAART era: a population-based study. AIDS. 2014;28:2313 CrossRef Pubmed
29. Barbaro G. Cardiovascular manifestations of HIV infection. J R Soc Med. 2001;94(8):384-390. doi:10.1177/014107680109400804. CrossRef Pubmed
Copyright Information
