Case Series
August 2024, 33:2
First online: 30 August 2024
Case Series
NSTEMI in 50-year-old Filipino Male with an Absent Left Main Coronary Artery and Left ACAOS with Intraarterial Course: A Very Rare Encounter
Renato C. Ong, Jr., RMT, M.D., FPCP, FPCC,1 Valerie R. Zarza-Geron, MD, FPCP, FPCC, FPSE, 1 Charles Tadeo O. Galang, MD, FPCP, FPCC 1
Main author contact information: r.ongjrmd@gmail.com
Address for correspondence: Renato C. Ong Jr., MD. Section of Cardiology Makati Medical Center 6FT2 2 Amorsolo Street, Legaspi Village, Brgy. San Lorenzo, Makati City, Philippines 1229.
Mobile No.: +639171859797
Telephone No.: +6388888999
ABSTRACT
Coronary artery anomalies are extremely uncommon occurrences. Encountering a combination of an absent left main coronary artery (LMCA) and a left anomalous origin of the coronary artery from the opposite sinus of Valsalva (ACAOS) is an exceptionally rare phenomenon. In this report, we present a case of a 50-year-old Filipino male who was diagnosed with a congenital absence of the LMCA and left ACAOS with an interarterial course, and subsequently experienced a non-ST elevation myocardial infarction (NSTEMI).INTRODUCTION
Coronary artery anomalies, which can be classified based on their origin, course, or termination, are congenital disorders with various manifestations that can range from benign to life-threatening.1-5 These anomalies have a prevalence of 0.26% in the general population5-7 and can present with a wide range of symptoms such as cardiomyopathies, chest pain, heart failure, arrhythmias, myocardial infarction, sudden cardiac death, or syncope.3-6
We present a 50-year-old Filipino male who had a congenital absence of the left main coronary artery (LMCA) and a left coronary artery originating from the opposite sinus of Valsalva with an interarterial course (left ACAOS IAC). This patient subsequently developed non-ST elevation myocardial infarction (NSTEMI).
CASE
A 50-year-old Filipino male diabetic came in due to chest pain with associated 10-day history of easy fatigability and 2-3 pillow orthopnea. Upon arrival at the ED, he was hemodynamically stable, and the physical examination was unremarkable. ECG showed sinus rhythm with nonspecific ST-T wave changes (Figure 1). Biochemical tests showed an NT-ProBNP of <60 pg/ml and a 36x elevated high sensitivity troponin I than normal. A transthoracic echocardiogram (TTE) showed normal left ventricular dimension with normal wall motion and contractility and a normal ejection fraction of 65%. Low molecular weight heparin, dual antiplatelets and statin therapy were started.
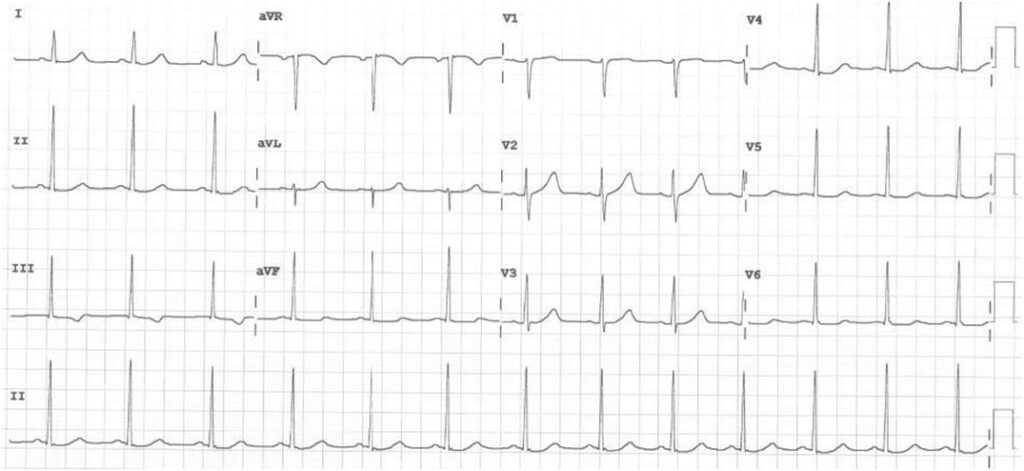
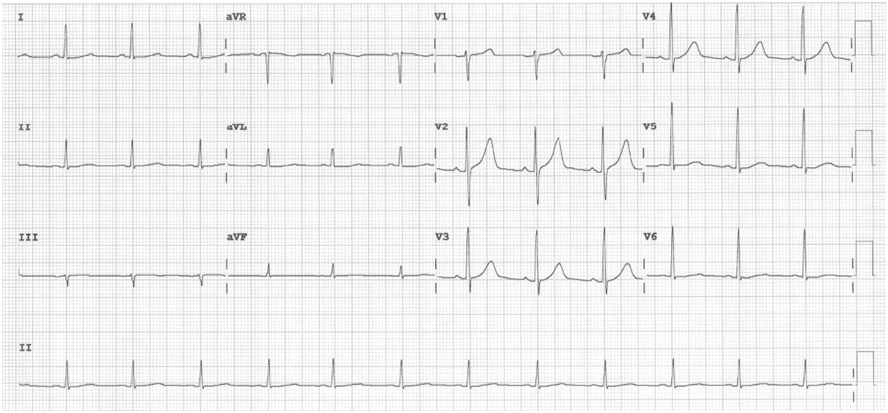
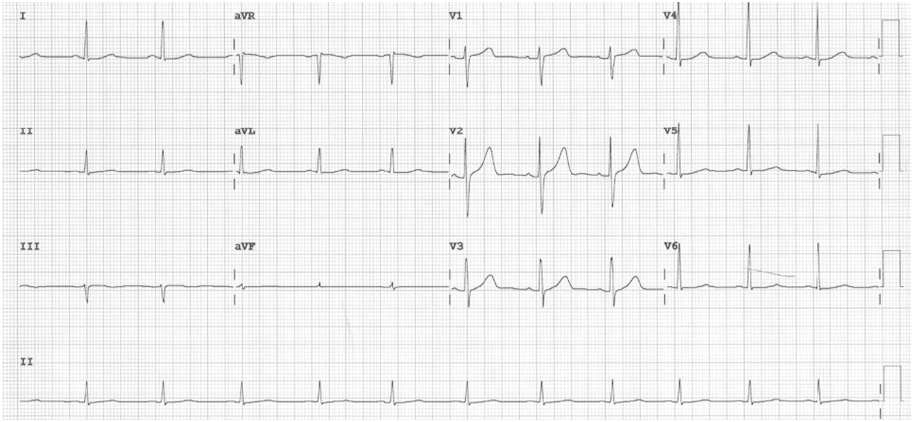
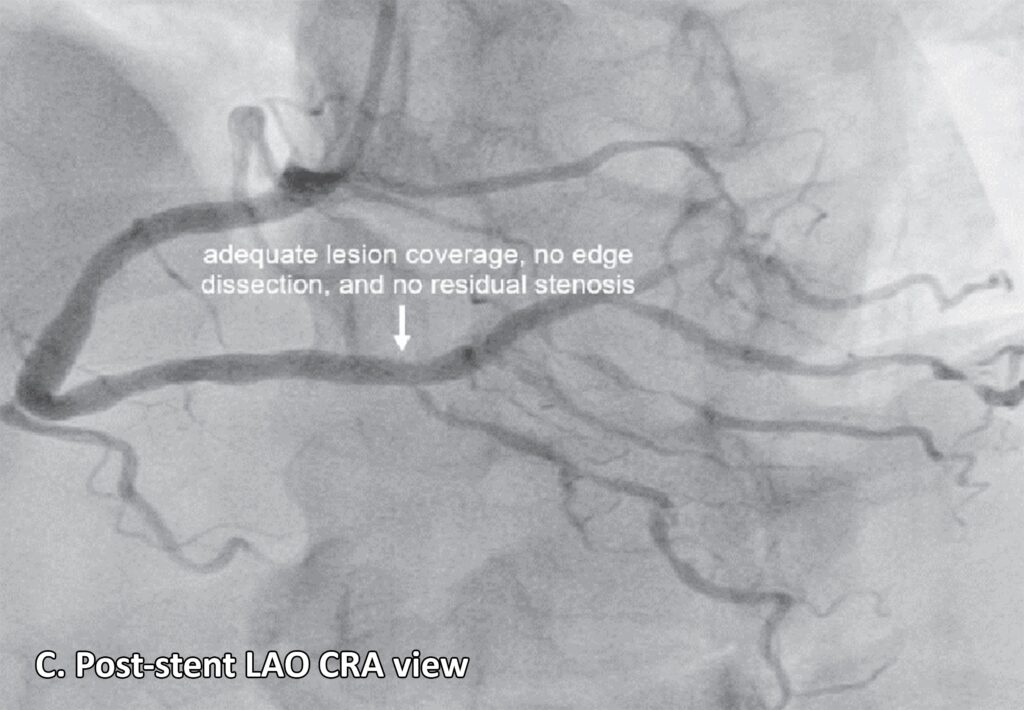
A left heart catherization with coronary angiography showed an absent left main coronary artery, a left circulation originating near the ostium of the right coronary artery (RCA): a left anterior descending artery (LAD) and a left circumflex artery (LCX) with mild luminal irregularities, and a 2 mm ramus intermedius with mild to moderate luminal irregularities. A large-sized RCA with 95-99% focal and eccentric stenosis at the distal segment prior to giving off the posterior descending artery which has a 60-70% ostial stenosis and several posterolateral branches (PLB), with the 2nd PLB having 80-95% stenosis at the proximal segment (Figure 2). Successful percutaneous transluminal coronary angioplasty of the RCA lesion was done using an everolimus-eluting platinum chromium coronary stent (Figure 3). He was discharged on dual anti-platelet therapy with improved clinical profile.
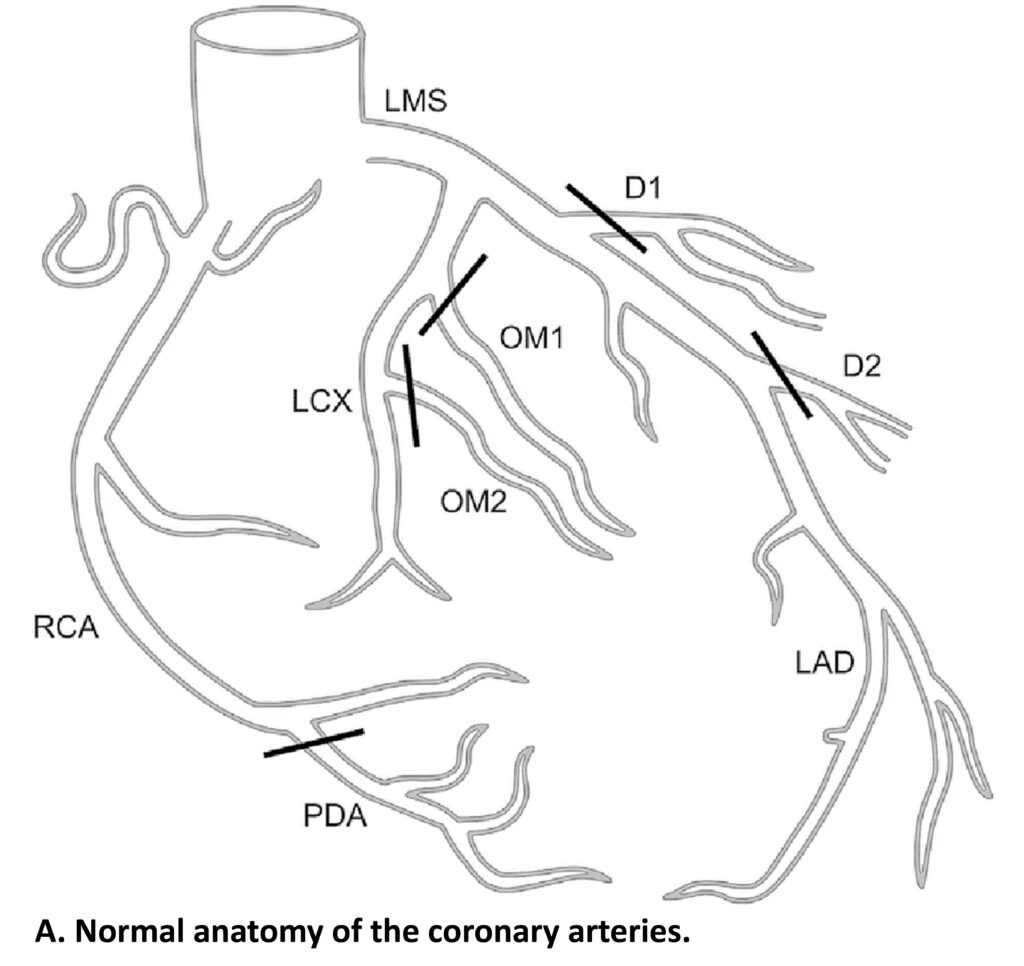

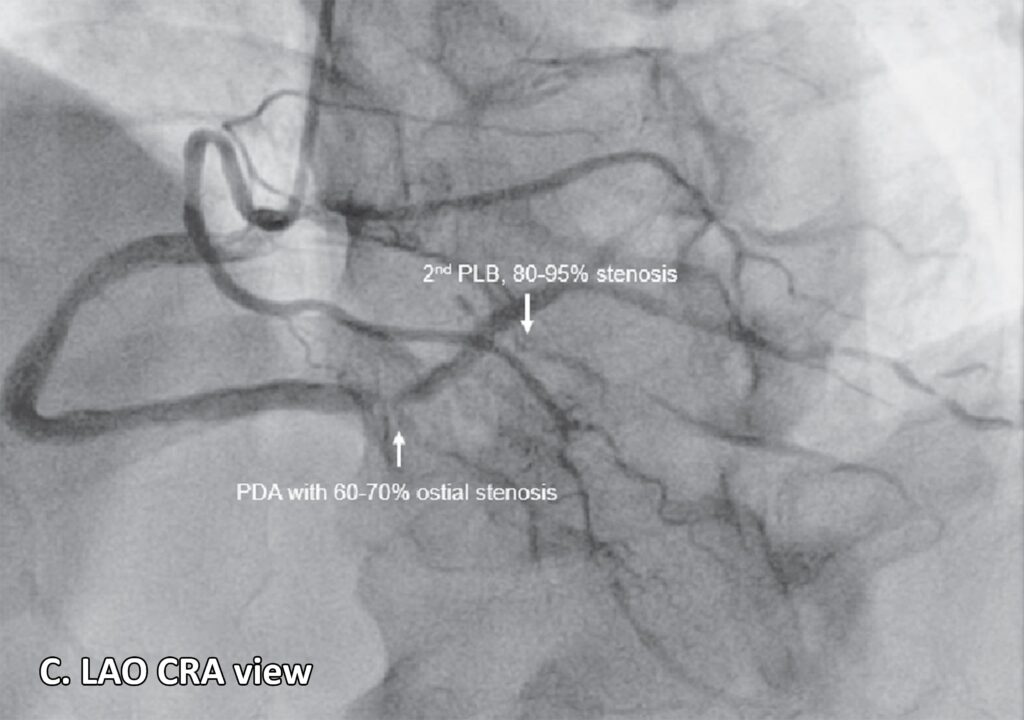
Figure 2: Coronary angiogram. A. Normal anatomy of the coronary arteries21 (lifted from Zakkaroff, Constantine & Biglands, John & Greenwood, John & Plein, Sven & Boyle, Roger & Radjenovic, Aleksandra & Magee, Derek. Patient-specific coronary blood supply territories for quantitative perfusion analysis. Computer Methods in Biomechanics and Biomedical Engineering: Imaging & Visualization. 2016;6:1-18.) B. LAO CAU view shows an absent LMCA. The left circulation seems to originate near the ostium of the RCA: a 2mm LAD with mild diffuse luminal irregularities, a 2 mm LCX with mild luminal irregularities, giving rise to several terminal obtuse marginal branches with mild to moderate luminal irregularities, a 2 mm ramus intermedius with mild to moderate luminal irregularities. A 4.5 mm RCA with 95-99% focal and eccentric stenosis at the distal segment prior to giving off the PDA. C. LAO CRA view shows a PDA with 60-70% ostial stenosis with mild to moderate luminal irregularities along the proximal to mid segment. The RCA gives off several PLB, with the 2nd PLB having 80-95% stenosis at the proximal segment and the remainder with minimal luminal irregularities.
Legend: LAO CAU, left anterior oblique caudal; LMCA, left main coronary artery; RCA, right coronary artery; LAD, left anterior descending artery; LCX, left circumflex artery; PDA, posterior descending artery; LAO CRA, left anterior oblique cranial; PLB, posterolateral branches.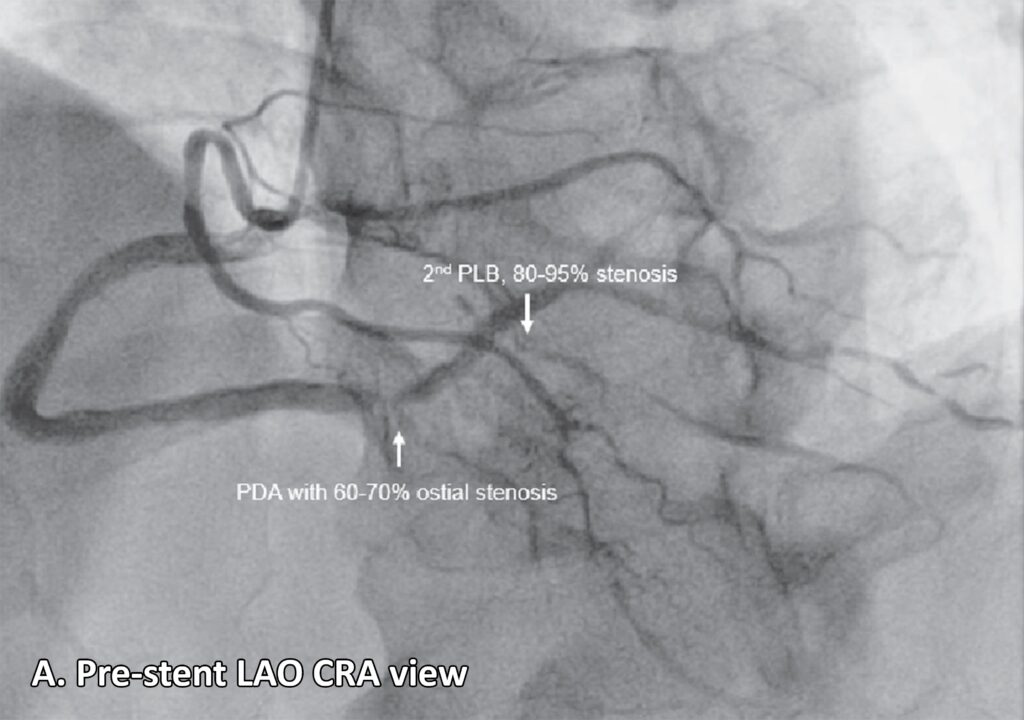

DISCUSSION
To identify relevant studies, a systematic review and search of the MEDLINE, EMBASE and Google Scholar databases was conducted using the following search terms: (‘coronary artery anomaly’ OR ‘ACAOS’ OR ‘absent left main coronary artery’) AND (‘acute coronary syndrome’ OR ‘NSTEMI’). Using the same approach, research articles were identified from reference lists of reviewed articles as well as by searching journals’ websites and hand searching. Inclusion criteria stipulated that the full text of papers be in English.
The coronary arteries start from the aorta and converge towards the heart’s apex. It divides into two primary branches – the RCA and the LMCA, which immediately bifurcate into LAD and LCX. These arteries provide blood supply to the major regions of the left ventricle and the interventricular septum.6, 8-9
The incidence of left coronary artery originating from the opposite sinus of Valsalva with an interarterial course (left ACAOS IAC) stands at 0.03%,7 and it has been tied to cases of sudden cardiac death, especially in young patients participating in vigorous physical activity.10-13 It has also been associated with arrhythmias and myocardial ischemia, even in the absence of arteriosclerosis.4, 13 The precise mechanism linking left ACAOS to adverse cardiac events remains unclear.7 An absent LMCA is a rare anomaly, with a prevalence of less than 0.5%.4, 9, 13-15 This anomaly can present with either benign or fatal complications later in life.4 When the LMCA, LAD, and LCX arise separately from the aortic sinus, it represents an exceedingly rare occurrence (Figure 4).9, 16 And finding the amalgamation of these two is exceedingly rare.
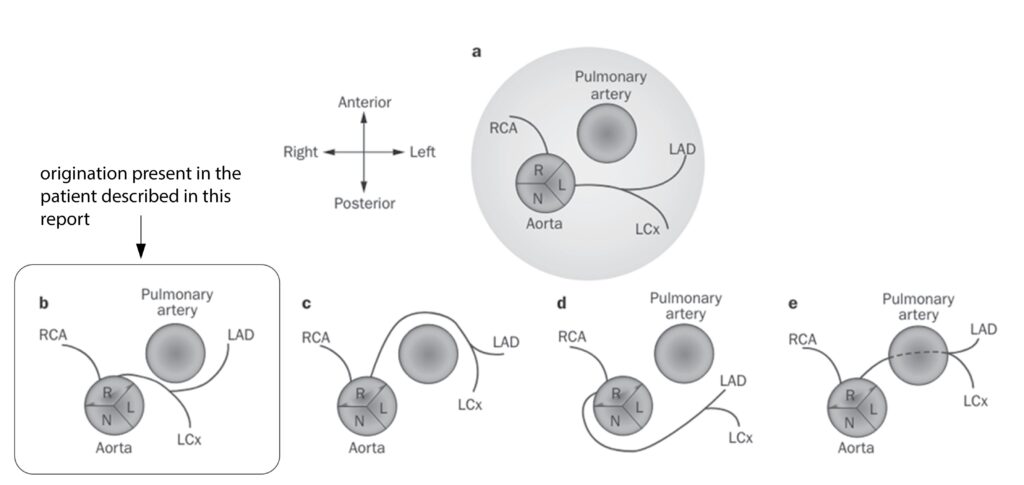
Figure 4: Anomalous origination of the left coronary artery from the right coronary sinus: variations in the course of the anomalous artery.22 (origination present in the patient described in this report). c. ACAOS with a prepulmonary course. d. ACAOS with a retroaortic course. e. ACAOS with a subpulmonic course. (lifted from Lim J, Beale A and Ramcharitar S. Nat. Rev. Cardiol. 2011:8; 706–19.)
Legend: ACAOS, anomalous origination of a coronary artery from the opposite sinus; L, left coronary sinus; LAD, left anterior descending coronary artery; LCx, left circumflex artery; N, noncoronary sinus; R, right coronary sinus; RCA, right coronary artery.The macroanatomic structural characteristics that contribute to ischemia, scarring, and pathological arrhythmias have been found to play a significant role in the recurrence of coronary insufficiency, as suggested by several studies.17-18 During physical exercise, the combination of tachycardia and hemodynamic overload can lead to an increased demand on the myocardium and a decrease in coronary flow. This can be attributed to various pathophysiological mechanisms, including the kinking of an anomalous vessel at an acute take-off angle, intermittent closure of the slit-like ostium, proximal narrowing with an elliptic vessel shape and intramural course, lateral compression of the aorta on the intramural segment, dynamic compression of the interarterial segment by the aorta and pulmonary artery, and an increased vulnerability to coronary vasospasm.7, 13, 17, 19-20
This gentleman with diabetes was admitted for chest pain, along with easy fatigability and orthopnea. Elevated cardiac biomarkers confirmed acute coronary syndrome. Heart failure symptoms were probably due to the dynamic compression of the interarterial segment by the aorta and pulmonary artery, linked to the cytoarchitectural abnormal coronary arteries. Whereas the chest pain was driven by ischemia from atherosclerosis.
A combination of imaging modalities is frequently essential to acquire sufficient data on anatomical high-risk features (such as multidetector CT scan, coronary CT angiography), ischemia, and concurrent coronary artery disease (including electrocardiogram, transthoracic echocardiogram, invasive coronary angiography), as well as indications of possible myocardial fibrosis/scar as a substrate for ventricular tachyarrhythmias (cardiac MRI).6, 13, 15 In this case, the coronary anomalies were incidentally detected during left heart catheterization with coronary angiogram.
Treatment options for patients with left ACAOS with IAC involve surgical correction or “unroofing” to correct dynamic lateral compression of the arterial wall, particularly in cases with an intramural segment. Other options include percutaneous coronary intervention, as well as medical and conservative treatment.15 In our case, the patient underwent percutaneous transluminal coronary angioplasty of the RCA using an everolimus-coated coronary stent.
CONCLUSION
While infrequently encountered, coronary artery anomalies manifest with highly variable clinical symptoms, and the exact pathophysiological mechanism underlying them is still not well understood. The utilization of angiographic detection is indispensable for assessing the significance of these findings and formulating appropriate management strategies.
Keywords: Coronary artery anomaly, ACAOS, Absent left main coronary artery, Acute coronary syndrome, NSTEMI.
REFERENCES
1. Ayaji NO, Lazarus L, Vanker EA and Satyapal KS. Absent Left Main Coronary Artery with Variation in the Origin of its Branches in a South African Population. Anat. Histol. Embryol. 2015;44:81–85. CrossRef Pubmed
2. Majid Y, Warade M, Sinha J, Kalyanpur A, and Gupta T. Superdominant right coronary artery with absent left circumflex artery. Biomed. Imaging Interv. J. 2011; 7, e2. CrossRef Pubmed
3. Ashraf S, Salman SH, Ali N, Kulshreshtha S, and Saad M. A Rare Presentation of Angina and Arrhythmia in Absent Left Main Coronary Artery. Cureus. 2020 Dec; 12(12): e12142. CrossRef Pubmed
4. Danias PG, Stuber M, McConnell MV, and Manninga WJ. The diagnosis of congenital coronary anomalies with magnetic resonance imaging. Coron. Artery Dis. 2001;12,621–626. CrossRef Pubmed
5. Yamanaka O and Hobbs RE. Coronary artery anomalies in 126,595 Patients undergoing coronary arteriography. Cathet. Cardiovasc. Diagn. 1990;21, 28–40. doi: 10.1002/ccd.1810210110. CrossRef Pubmed
6. Yilmaz-Cankaya B, Kantarci M, Yalcin A et al. Absence of the Left Main Coronary Artery: MDCT Coronary Angiographic Imaging. Eurasian J Med. 2009 Apr; 41(1): 56–58. Pubmed
7. Bigler MR, Ashraf A, Seiler C, et al. Hemodynamic Relevance of Anomalous Coronary Arteries Originating from the Opposite Sinus of Valsalva-In Search of the Evidence. Front. Cardiovasc. Med., 21 January 2021. CrossRef Pubmed
8. Kardos A, Babai L, Rudas L, et al. Epidemiology of congenital coronary artery anomalies: a coronary arteriography study on a central European population. Cathet Cardiovasc Diagn. 1997;42:270–275. CrossRef Pubmed
9. Kastellanos S, Aznaouridis K, Vlachopoulos C, Tsiamis E, Oikonomou E, Tousoulis D. Overview of coronary artery variants, aberrations and anomalies. World J Cardiol. 2018;10:127–140. CrossRef Pubmed
10. Camarda J, Berger S. Coronary artery abnormalities and sudden cardiac death Pediatr Cardiol. 2012 Mar;33(3):434-8. doi: 10.1007/s00246-012-0168-0. Epub 2012 Feb 10. CrossRef Pubmed
11. Cheitlin, MD, De Castro CM and McAllister HA. Sudden death as a complication of anomalous left coronary origin from the anterior sinus of valsalva. A not so minor congenital anomaly. Circulation. 1974;50,780–7. CrossRef Pubmed
12. Taylor AJ, Rogan KM and Virmani R. Sudden cardiac death associated with isolated congenital coronary artery anomalies. J. Am. Coll. Cardiol. 1992;20,640–7. CrossRef Pubmed
13. Basso C, Maron BJ, Corrado D and Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J. Am. Coll. Cardiol. 2000;35,
1493–1501. CrossRef Pubmed
14. Baim DS, Grossman W. Coronary angiography Cardiac Catheterization Angiography, and Intervention. 5th ed. Baltimore, MD: Williams & Wilkins; 1996;183–208.
15. Grani C, Kaufmann PA, Windecker S, Buechel RR. Diagnosis and Management of Anomalous Coronary Arteries with a Malignant Course. Interv Cardiol. 2019 May; 14(2): 83–88. CrossRef Pubmed
16. Cheezum MK, Liberthson RR, Shah NR, Villines TC, O’Gara PT, Landzberg MJ, Blankstein R. Anomalous aortic origin of a coronary artery from the inappropriate sinus of Valsalva. J Am Coll Cardiol. 2017;69:1592–1608. CrossRef Pubmed
17. Davis JA, Cecchin F, Jones TK and Portman MA. Major coronary artery anomalies in a pediatric population: Incidence and clinical importance. J. Am. Coll. Cardiol. 2001:37,593–597. CrossRef Pubmed
18. Maron BJ, Haas TS, Ahluwalia A et al. Demographics and epidemiology of sudden deaths in young competitive athletes: from the United States National Registry. Am J Med. 2016;129:1170–7. doi: 10.1016/j.amjmed.2016.02.031. CrossRef Pubmed
19. Lorenz EC, Mookadam F, Mookadam M et al. A systematic overview of anomalous coronary anatomy and an examination of the association with sudden cardiac death. Rev Cardiovasc Med. 2006;7:205–13. Pubmed
20. Eckart RE, Scoville SL, Campbell CL et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–34. doi: 10.7326/0003-4819-141-11 200412070-00005. CrossRef Pubmed
21. Zakkaroff, Constantine & Biglands, John & Greenwood, John & Plein, Sven & Boyle, Roger & Radjenovic, Aleksandra & Magee, Derek. Patient-specific coronary blood supply territories for quantitative perfusion analysis. Computer Methods in Biomechanics and Biomedical Engineering: Imaging & Visualization. 2016;6:1-18. doi: 10.1080/21681163.2016.1192003. CrossRef Pubmed
22. Lim J, Beale A and Ramcharitar S. Anomalous origination of a coronary artery from the opposite sinus. Nat. Rev. Cardiol. 2011:8; 706–19. doi:10.1038/nrcardio.2011.147. CrossRef Pubmed
Copyright Information
