Case Report
August 2024, 33:2
First online: 30 August 2024
Case Report
Case Report: Downsizing Patent Ductus Arteriosus Stent Using a Modified Microvascular Plug
Kelvin Goh Leong Hoe,1 Shereen Toh May Yi,1 Mohammad Tamim bin Jamil,1 Koh Ghee Tiong2
2 Department of Pediatric Cardiology, Hospital Sultan Idris Shah, Serdang, Malaysia
Main Author: Kelvin Goh Leong Hoe
Email Address: klvn0726@gmail.com
ABSTRACT
Maintaining patency of the ductus arteriosus is crucial for ductdependant congenital heart diseases while awaiting definitive interventions. However, it may be challenging to balance systemic and pulmonary blood flow (PBF) when placing a Patent Ductus Arteriosus (PDA) stent. There is currently limited data when it comes to successful transcatheter techniques in treating over-shunting. We describe a successful application of a modified Medtronic Microvascular Plug (MVP)™ System to effectively treat a case of PDA stent over-shunting in a onemonth-old infant with critical Pulmonary Stenosis (PS). KeywordsPDA stent, Overshunting, Microvascular plug (MVP), Transcatheter
INTRODUCTION
In infants with duct-dependant circulations, maintaining the patency of the ductus arteriosus is crucial while awaiting further definitive intervention. However, it may be challenging to balance systemic and pulmonary blood flow (PBF) when placing a Patent Ductus Arteriosus (PDA) stent. Oversized PDA stents may result in over-shunting that may not respond to medical therapies. Surgical options such as clips or bands are less favorable due to the risk of stent distortion and damage. There is currently limited data when it comes to successful transcatheter techniques in downsizing PDA stents.
The Medtronic Microvascular Plug (MVP)™ System (MVP, Medtronic, Minneapolis, MN, USA) is designed with the intention to obstruct or reduce the rate of blood flow in the peripheral vasculature. By removing sections of its polytetrafluoroethylene (PTFE) cover, the MVP system has been used to reduce blood flow in branch pulmonary arteries in various congenital heart diseases via the transcatheter approach. However, there are very limited reports on its usage in treatment of over-shunting in patients post PDA stenting.
We describe a successful application of a modified MVP to effectively treat a case of PDA stent over-shunting in a one-month-old infant with critical Pulmonary Stenosis (PS).
CASE REPORT
LL was born at 37 weeks via spontaneous vaginal delivery, with a birth weight of 2.56kg. She presented with cyanosis at birth and was initially diagnosed with pulmonary atresia with intact ventricular septum (PAIVS) with a tripartite right ventricle (RV). She was started on intravenous Prostaglandin and was planned for pulmonary valve perforation with a plan for PDA stenting if needed. However, the procedure was delayed until Day 10 of life due to necrotizing enterocolitis.
She underwent cardiac catheterization at Day 10 of life which revealed a tripartite right ventricle, presence of minimal forward flow from RV to pulmonary artery (PA) and a large 3.5mm PDA. The pulmonary valve (PV) annulus measured 4.3mm. Percutaneous Balloon Valvuloplasty (PTBV) of the PV was then performed. Post PTBV angiogram showed good forward flow across the PV with dynamic infundibular pulmonary stenosis. Echocardiogram (ECHO) post procedure showed mild Pulmonary Stenosis (PS) with peak gradient of 25mmHg, Tricuspid Regurgitation (TR) with peak pressure gradient of 60 mmHg and generous flow across the PDA.
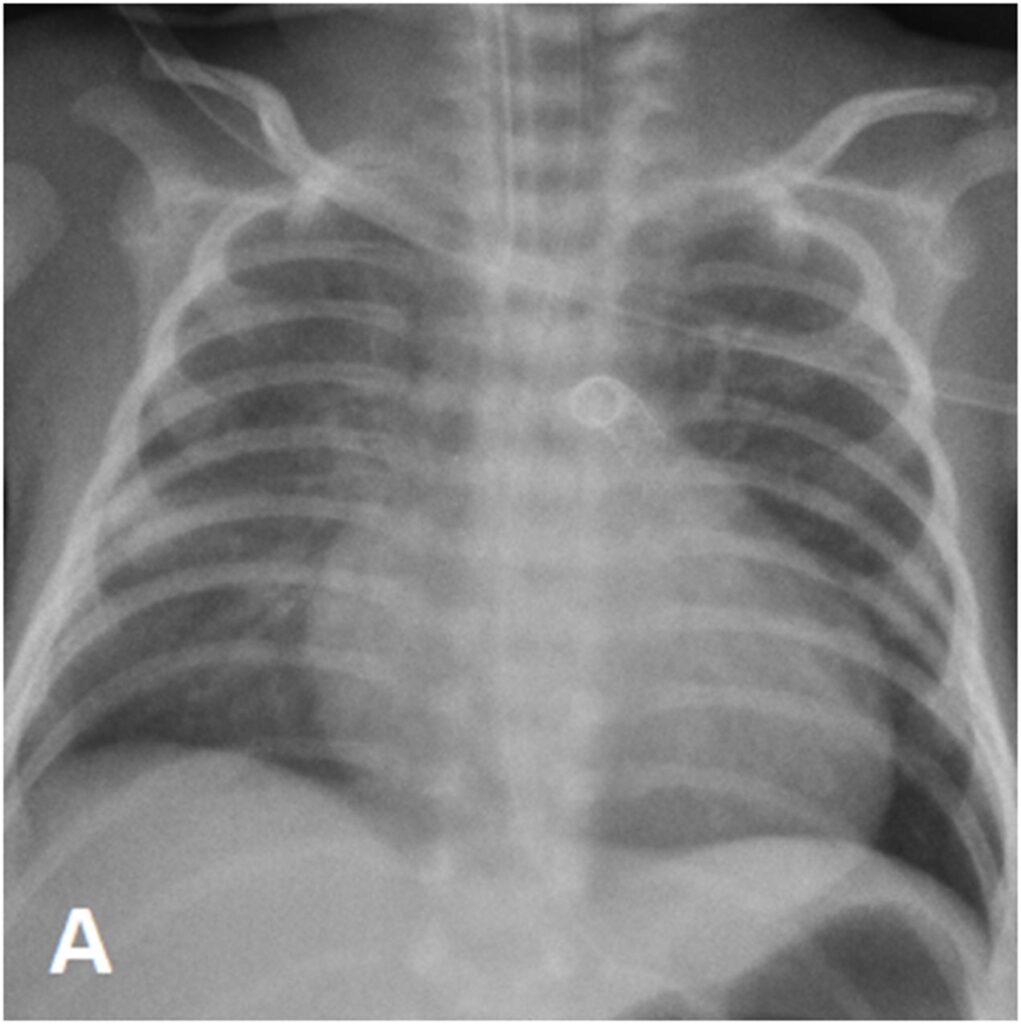
She was extubated three days post procedure and Prostaglandin was weaned off two days later. She was reintubated two days after discontinuation of Prostaglandin due to respiratory distress with desaturations. ECHO showed a constricted PDA measuring 2mm with moderate PS with peak gradient of 43mmHg and TR gradient of 61mmHg. She underwent emergency PDA stenting on the same day. The PDA was stented with a 4mm x 19mm RX Angiolite iVascular coronary stent (iVascular, Barcelona, Spain).
Subsequently, LL developed signs of PDA overshunting which responded to a combination of medical therapy and ventilation strategies. However, she remained ventilator-dependent. ECHO showed mild PS with peak gradient of 29mmHg, TR with a gradient of 66mmHg and generous flow across the PDA stent. Thus, prompting the decision to downsize the PDA stent transcatheterly.
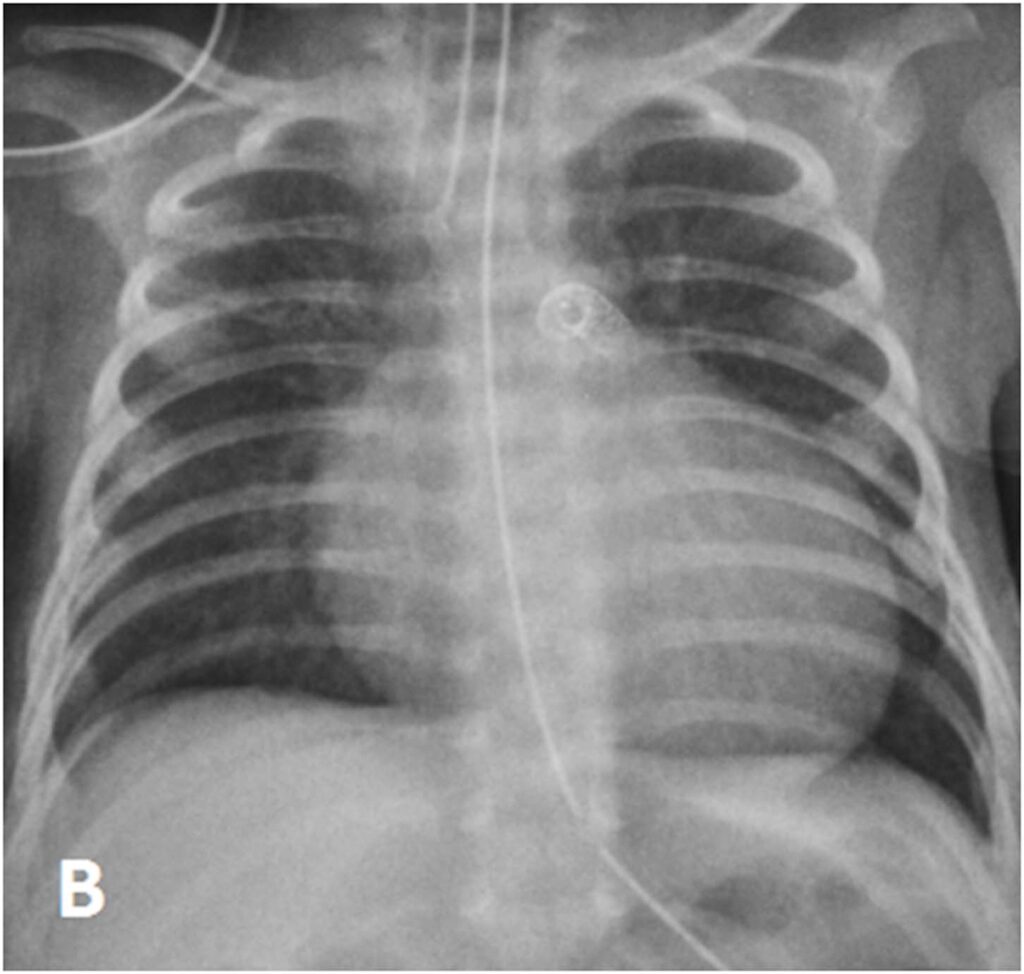
At day 29 of life, she underwent downsizing of the PDA stent. Using a 23G hypodermic sterile needle, three fenestrations was made into three of the eight covered cells of a 6.5mm x 12mm Microvascular plug (MVP-5Q). The device was then deployed at the centre of the PDA stent via a 4Fr Glidecath® (Radifocus Glidecath; Terumo, Tokyo, Japan) catheter to limit blood flow across the stent. Post-device deployment angiogram showed reduced flow across PDA stent and saturations decreased from 99% to 90%. Post-procedure ECHO showed residual PS with peak gradient of 13mmHg, TR of 40mmHg and restricted flow across PDA stent with peak systolic gradient of 70mmHg. There was also an improvement of diastolic blood pressure by 10-15mmHg post procedure.
The procedure was successful and she was successfully extubated four days post-procedure. She was discharged home well on room air at Day 48 of life.
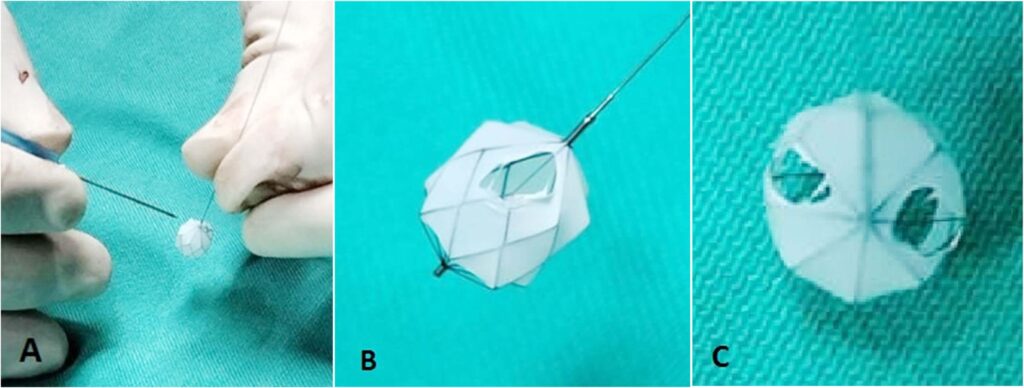
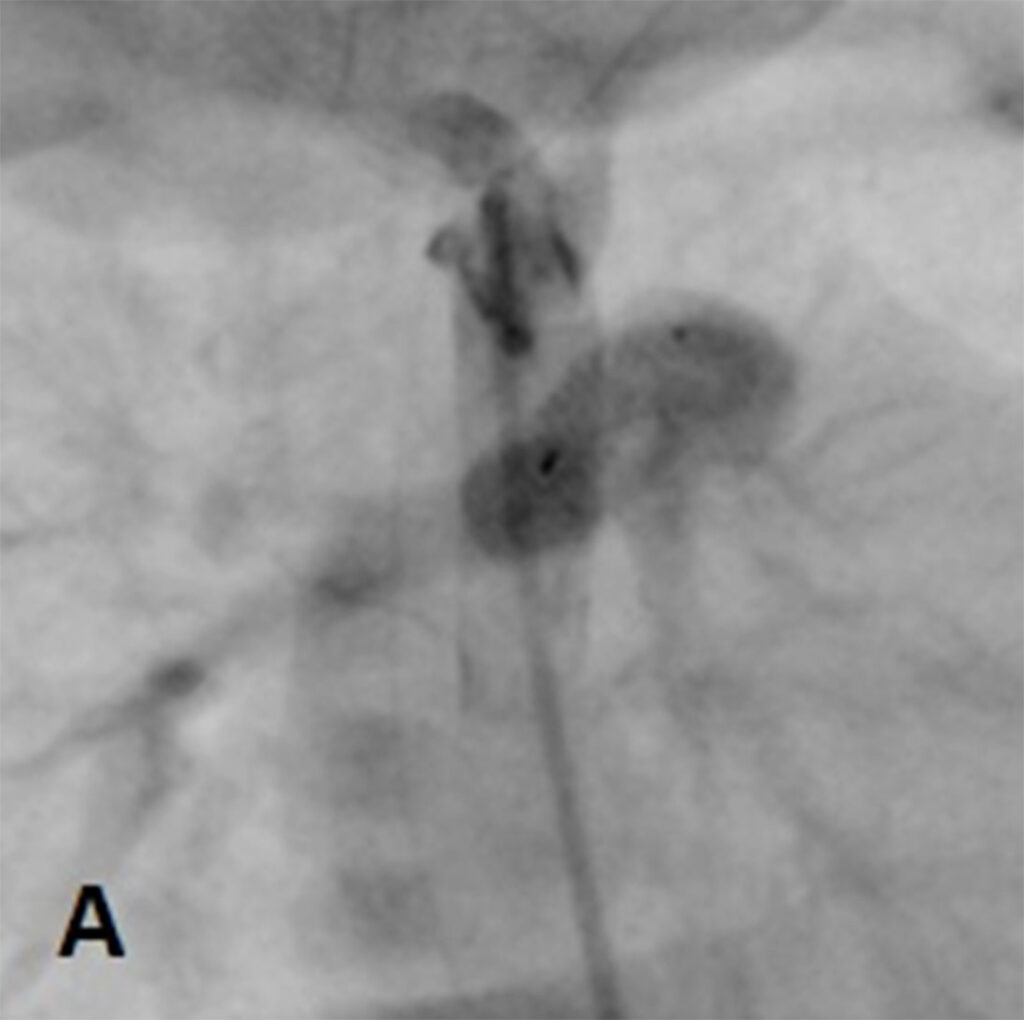
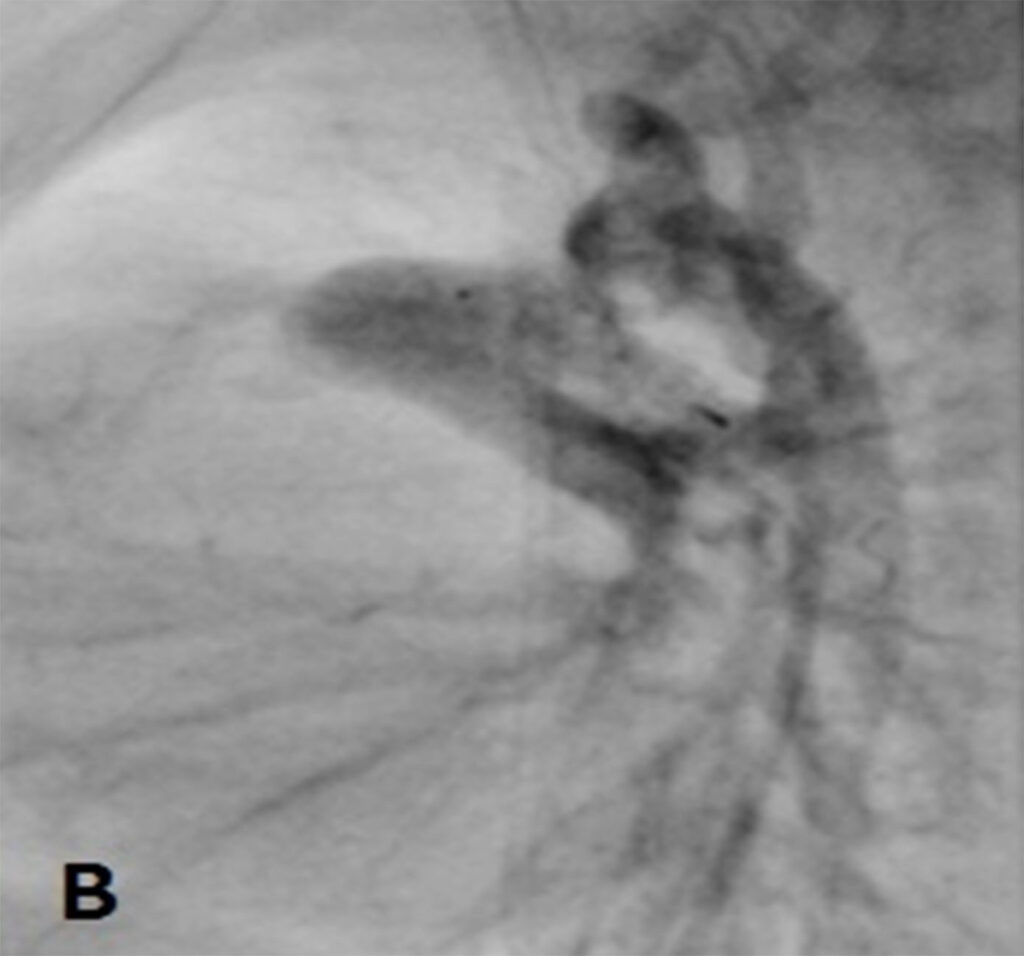
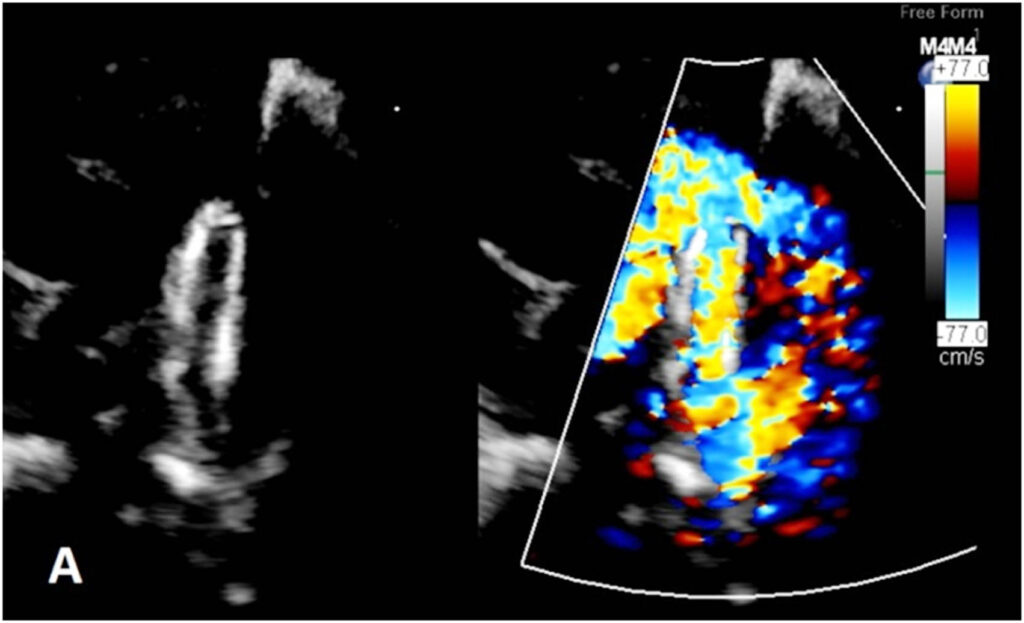
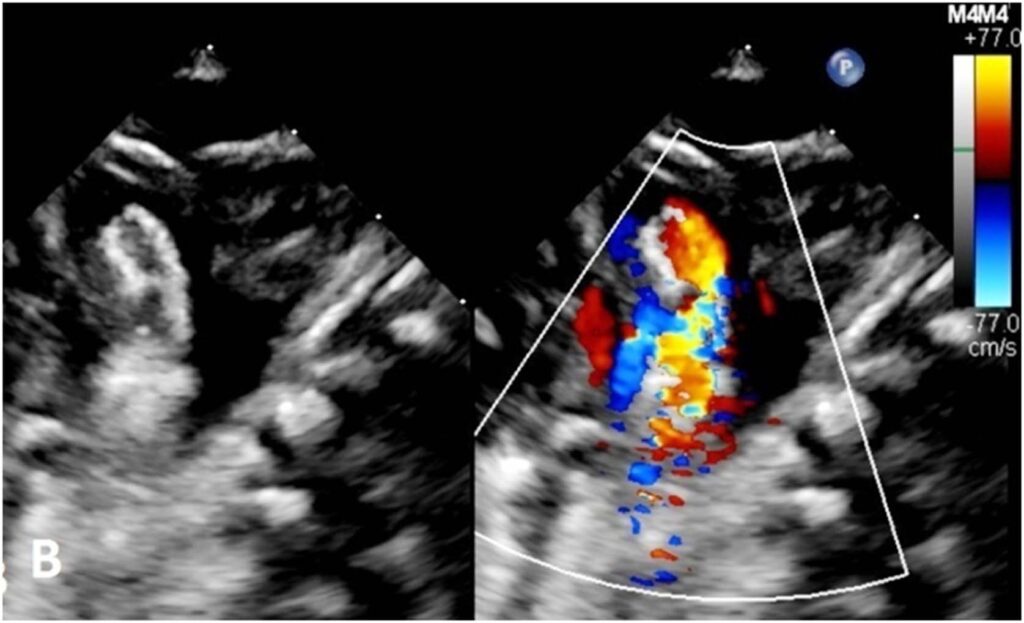
Figure 3:(A) Echocardiogram before deployment of the modified MVP-5Q showed generous flow across the PDA stent compared to (B) restricted flow after deployment of the modified MVP-5Q within the stent.
DISCUSSION
Selecting the appropriate PDA stent size is essential to prevent post stenting complications such as stenosis, stent migration and in this case, over shunting. Alwi et al. in their study recommends PDA stent diameters of 3.5mm for patient weighing less than 3kg, 4mm for those 3-5kg and 4.5mm in those weighing more than 5kg.1 In our case, LL weighed 2.88kg at the point of stenting. However, as the PDA measured 3.5mm in diameter, there were concerns regarding stent migration and thus a 4mm stent was selected, which may have contributed to the overshunting.
Medical management of symptomatic over-circulation in post-PDA stent patients may sometimes be inadequate whereas surgical reduction such as ligation and clips carry the risk of additional sternotomy as well as stent distortion. Transcatheter option using modified MVPs has been well described to reduce blood flow across branched pulmonary arteries but has yet to be sanctioned for the use in downsizing PDA stents.2 Maschietto N et al has described a novel technique placing a modified Diabolo stent within the PDA stent but this requires a 6F delivery system which may not be suitable in smaller babies.3
Our case highlights the positive outcome of using a modified MVP as a flow restrictor in PDA stent overshunting. It can be modified easily, requires only low profile delivery catheters, easily retrievable and reposition and offers immediate mechanical obstruction/restriction to the flow rather than relying on thrombus formation allowing us to see its immediate effects post deployment and make necessary modifications if necessary prior to release.4 To date, the modified MVP has been largely found to be successful as a flow limiter in the main and branched pulmonary arteries in multiple case series and animal studies.5 To our knowledge, there is only one other report of a modified MVP being used as a flow restrictor in PDA stent overshunting. Lauren N et al had described similar success in using a modified MVP as a flow restrictor.6
There has been very limited data as to what extent of predeployment modification is needed to achieve desired flow restriction. We first created three fenestrations to the 5Q MVP using a 23G hypodermic sterile needle, targeting a flow reduction of approximately 50% through the stent’s crosssectional area, though mathematically, removing 3 out of 8 covered cells will only result in 37.5% reduction of cross sectional area, and found the post deployment results to be desirable. Creation of limited fenestration initially will allow for further modification to the same device until the desired results are obtained as there is no quantitative measurement pre-deployment that reflects the exact amount of flow reduction. Rather, surrogate readings of improvement in diastolic blood pressure, reduction in oxygen saturations and subjective flow reduction from ECHO and angiogram pre and post deployment of the implant serve as a guide as to whether the modifications made was adequate prior to releasing the device.6, 7
Few techniques of modifications have been mentioned, including using blade scalpel, eye Bowie and coronary balloons for more uniform dilatation.6, 7 But neither has proven superior to the other in creating a desired fenestration mainly because it is almost impossible to determine the ideal size of fenestration to achieve the desired outcome. In our case, the 23G hypodermic sterile needle was chosen as it is readily available, the needle hub provided sufficient grip and easy maneuverability and the uniform needle shaft with a short bevel avoided unintentional over-dilatation of the MVP PTFE sections. The end results were comparable to other modification techniques but further studies needs to be done in the future to determine the optimal modification technique.
There were no immediate complications related to the procedure or placement of the MVP within the stent. Risk of thrombus formation at the MVP and complete occlusion of the PDA stent can be minimized with adequate antiplatelet therapy.7
CONCLUSION
The prospect of using a modified MVP to act as a flow restrictor in treating PDA stent overshunting is promising, with a good safety profile and positive desirable outcome. With further studies into the various techniques to modify the device, it may be possible to predetermine the type and extent of modification needed prior to the procedure. Further follow-up will be needed to determine the mid-term outcome and complications related to the procedure.
Author contributions
All authors were involved in the clinical care and procedures performed on the patient. KLH Goh took the lead in writing and revising the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgement
The authors would like to acknowledge Dr HS Koay for his role in the clinical care and procedures of the patient.
Conflict of interest and financial disclosure
None.
Ethics statement and patient consent
The parents underwent informed procedural consent prior to the procedure. Written informed consent was obtained from parents for the purpose of this publication.
REFERENCES
1. Alwi, M, Choo, K, Latiff, H. et al. Initial results and medium-term follow-up of stent implantation of patent ductus arteriosus in duct-dependent pulmonary circulation. J Am Coll Cardiol. 2004 Jul, 44 (2) 438–445. https://doi.org/10.1016/j.jacc.2004.03.066 CrossRef Pubmed
2. Nageotte, S., Shahanavaz, S., Eghtesady, P., & Balzer, D. (2021). Total Transcatheter Stage 1: A Word of Caution. Pediatric cardiology, 42(6), 1410–1415. https://doi.org/10.1007/s00246-021-02626-w CrossRef Pubmed
3. Maschietto, N., Baird, C., & Porras, D. (2020). Percutaneous intraluminal downsizing of systemic-to-pulmonary artery shunts: a novel application of the Diabolo stent technique-Case series and description of the technique. Catheterization and cardiovascular interventions: official Journal of the Society for Cardiovascular Angiography & Interventions, 95(3), 471–476. https://doi.org/10.1002/ccd.28598 CrossRef Pubmed
4. Pellerin, O., Maleux, G., Déan, C., Pernot, S., Golzarian, J., & Sapoval, M. (2014). Microvascular plug: a new embolic material for hepatic arterial skeletonization. Cardiovascular and interventional radiology, 37(6), 1597–1601. https://doi.org/10.1007/s00270-014-0889-y CrossRef Pubmed
5. Khan, A. H., Hoskoppal, D., Kumar, T. K. S., Bird, L., Allen, K., Lloyd, H., Knott-Craig, C. J., Waller, B. R., & Sathanandam, S. (2019). Utility of the Medtronic microvascular plug™ as a transcatheter implantable
and explantable pulmonary artery flow restrictor in a swine model. Catheterization and cardiovascular interventions: official Journal of the Society for Cardiovascular Angiography & Interventions, 93(7), 1320–1328. https://doi.org/10.1002/ccd.28162 CrossRef Pubmed
6. Carlozzi, L. N., Johnston, T. A., Joanes, T. K., Rubio, A. E., & Morray, B. H. (2023). Modified Medtronic Microvascular Plug Implantation to Reduce Pulmonary Blood Flow in Patients With a Shunted Single Ventricle. Journal of the Society for Cardiovascular Angiography & Interventions, 2(5). https://doi.org/10.1016/j.jscai.2023.101070 CrossRef
7. Haddad, R. N., Bentham, J., Adel Hassan, A., Al Soufi, M., Jaber, O., El Rassi, I., & Kasem, M. (2023). Outcomes of manually modified microvascular plugs to pulmonary flow restrictors in various congenital heart lesions. Frontiers in cardiovascular medicine, 10, 1150579. https://doi.org/10.3389/fcvm.2023.1150579 CrossRef Pubmed
Copyright Information
