Case Report
August 2025, 34:2
First online: 18 August 2025
Case Report
The Efficacy and Safety of Triple Antithrombotic versus Dual Antiplatelet for Acute Coronary Syndrome without Atrial Fibrillation: A Meta-analysis
Jerahmeel Aleson L. Mapili, MD,1 John Christopher A. Pilapil, MD,1 Lauro L. Abrahan IV, MD1
Main Author: Jerahmeel Aleson L. Mapili (+639175922627, jlmapili@up.edu.ph)
ABSTRACT
BACKGROUND
Acute Coronary Syndrome (ACS) remains as one the leading cause of morbidity and mortality. Several randomized clinical trials have shown the efficacy of direct oral anticoagulants (DOACs) on top of dual-antiplatelet therapy (DAPT) among patients with recent ACS but have shown increased bleeding risk. However, there have been newer studies which showed lower risk for bleeding. Hence, this meta-analysis will look into the efficacy and safety of triple antithrombotic therapy (TAT), DAPT plus DOAC, among patients with recent ACS without Atrial Fibrillation (AF).
METHODOLOGY
This study used a random-effects meta-analysis using RevMan 5.4. The risk of bias was assessed using Cochrane RoB2 and the certainty of evidence was assessed using GRADE.
RESULTS
This meta-analysis showed that, when analyzed overall, TAT when compared to DAPT reduced the risk of composite MACE (RR 0.88, 95% CI 0.81-0.96) and myocardial infarction (MI) (RR 0.88, CI 0.78-0.98) with a significant risk for any (RR 1.9, 95% CI 1.32-2.74) and major bleeding (RR 2.26, 95% CI 1.47-3.48). When analyzed using the lowest dose, TAT had no significant benefit in reducing risk of composite MACE (RR 0.90, 95% CI 0.82-1.00) and MI (RR 0.93, 95% CI 0.81-1.05) but still poses significant risk for any (RR 1.57, 95% CI 1.18-2.10) and major bleeding (RR 2.28, 95% CI 1.45-3.60).
CONCLUSION
Among patients with recent ACS without AF, TAT at best modestly reduces the risk for composite MACE and myocardial infarction but poses a significant risk for bleeding when compared to DAPT. Further studies have to be made using the newer agents with larger populations to ascertain the value of TAT in ACS without AF.
Keywords
Acute coronary syndrome, Triple antithrombotic, Direct oral anticoagulant
INTRODUCTION
Acute Coronary Syndrome (ACS) remains to be one of the leading causes of morbidity and mortality both locally and globally. Globally, nearly half of all deaths are attributable to ischemic heart disease and 12% of disability-adjusted life years (DALYs) lost annually are attributable to ischemic heart disease.1 In the Philippines, ischemic heart diseases including ACS remains the top cause of mortality according to the Department of Health (DOH) and Philippine Statistics Authority (PSA) at 18.5% of all deaths in 2022.2
Patients with recent ACS, despite being on recommended dual-antiplatelet therapy (DAPT) even after having undergone revascularization, are at risk of recurrent ischemic events.3, 4, 5 In recent years, there has been growing evidence regarding the use of direct oral anticoagulants (DOACs) among patients with atrial fibrillation who have undergone percutaneous coronary intervention for acute coronary syndrome.6, 7, 8, 9 It has been shown that there is reduction in ischemic events among patients with atrial fibrillation and a recent myocardial infarction treated with PCI albeit with a trend towards increased bleeding.
However, the evidence on the use of DOACs among patients with recent acute coronary syndrome without atrial fibrillation is sparse. It is believed however that anticoagulation on top of dual-antiplatelet therapy may play a role even in the absence of atrial fibrillation among patients with recent acute coronary syndrome owing to the fact that there is excess thrombin generation that persists beyond the acute presentation of acute coronary syndrome.10 In 2013, a meta-analysis by Oldgren et. al. was done on the intervention in question which has shown that triple antithrombotic therapy had a modest reduction in major adverse cardiovascular events but with substantial increase in bleeding.11 Since then, studies after 2013 further looked into newer DOACs with other mechanisms of action and have shown lower bleeding rates compared to previous studies.24, 25 Hence, this meta-analysis looked into the efficacy and safety of triple antithrombotic therapy, DAPT plus DOAC, among patients with recent acute coronary syndrome without atrial fibrillation.
OBJECTIVES
This meta-analysis aimed to assess the following:
- Efficacy of Triple Antithrombotic Therapy versus Dual antiplatelet Therapy in terms of reducing major adverse cardiovascular events, myocardial infarction, and stroke among patients with recent acute coronary syndrome without atrial fibrillation
- Safety of Triple Antithrombotic Therapy in terms of any and major bleeding among patients with recent acute coronary syndrome without atrial fibrillation
METHODOLOGY
Study Eligibility Criteria
Inclusion Criteria
Studies which met the following criteria were included in this meta-analysis: (1) Study design is a randomized controlled trial, (2) Intervention includes direct oral anticoagulant (regardless of mechanism of action) on top of dual antiplatelet therapy, (3) Control includes placebo on top of dual antiplatelet therapy (4) Population are adults diagnosed with recent acute coronary syndrome without atrial fibrillation, (5) Efficacy outcome reported includes major adverse cardiovascular events and its component outcomes, (6) Safety outcome reported should include bleeding (clinically relevant non-major bleeding, major bleeding, and fatal bleeding) using any standard criteria, and (7) Only articles published in the English language or with readily available translation.
Exclusion Criteria
Studies were excluded if: (1) Study design is not a randomized controlled trial, (2) not in the English language or published in recognized international journals, (3) population includes patients with stable coronary artery disease or chronic coronary syndrome, (4) population includes patients with atrial fibrillation (5) outcomes are others not specified in the inclusion criteria.
Literature Search Strategy
The meta-analysis was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.12 The study protocol also underwent technical review under the University of the Philippine Department of Medicine Technical Review Board (TRB) and underwent ethical review under the University of the Philippines Manila Research Ethics Board (UPMREB).
An electronic search was independently performed by two investigators (JAM and JCP) who were trained with Good Clinical Practice (GCP) from NIDA. The following search engines were used: PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and ClinicalTrials.gov from their dates of inception to May 2023. The following search terms were used: “direct oral anticoagulant”, “novel oral anticoagulant”, “triple antithrombotic”, “acute coronary syndrome”, “STEMI”, “STE-ACS”, “NSTEMI”, “NSTE-ACS”, as free text and/or as MeSH terms. Additionally, search terms for randomized trial and clinical trial were used in order to yield a maximally sensitive search.13, 14 In addition, the investigators independently reviewed the list of references from retrieved articles to identify additional potentially relevant studies.
After the initial electronic search, the two investigators (JAM and JCP) independently reviewed for duplicates to complete the identification phase. The screening phase was composed of reviewing titles and abstracts only. Studies were excluded during this screening phase if they did not meet the inclusion criteria. Studies which met the inclusion criteria but are identified to have at least one exclusion criteria were still included in the eligibility phase for the full-text review. After screening for titles and abstracts, a list of eligible studies underwent a full-text review. Studies which met the inclusion and did not meet the exclusion criteria were included in the final meta-analysis.
Any discrepancy between the independent literature searches were resolved by discussion to reach consensus. Consultation with the third investigator (LLA) was made in case these discrepancies were not resolved by consensus.
Data Extraction and Critical Appraisal
Two investigators (JAM and JCP) independently reviewed each included article for data extraction of all study variables as laid out below. Any discrepancy between the independent review was resolved by discussion to reach a consensus. Consultation with the third investigator (LLA) was made in case these discrepancies were not resolved by the two investigators by consensus.
The following details were extracted from each included study and tabulated: study design, characteristics of the study population, duration of follow-up, intervention and control in their specified dosing schedules and routes of administration, and the number of events pertaining to each of the selected efficacy and safety outcomes. The data was extracted from the full text articles including their appendices and supplementary files (if applicable).
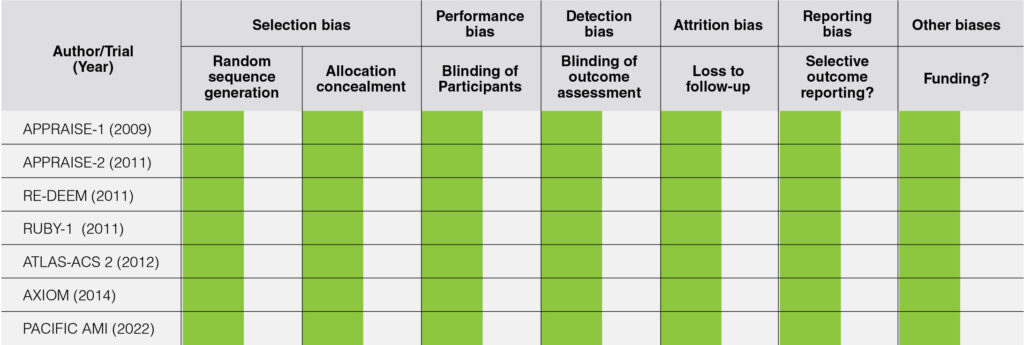
The risk of bias in each study was independently assessed by two investigators (JAM and JCP) using the Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB2), and was tabulated accordingly.15 The risk of bias table is color coded as follows: green for low risk, orange for unknown risk, and red for high risk; an explanation for the risk of bias is provided in the tabulation.
Overall confidence in the estimates for each outcome was independently assessed by two investigators (JAM and JCP) based on the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group system for limitations in study design, evidence directness, consistency, precision of results and publication bias.16
Data Synthesis and Statistical Analysis
All statistical analyses were conducted using Review Manager version 5.4 from Cochrane.17 Relative Risk (RR) was used as a summary statistic for dichotomous outcomes. The results of each study are presented in the Forest plots with summary statistics, 95% confidence intervals and relative weights represented by the middle of the square, the horizontal line, and the relative size of the square, respectively.
For the overall summary statistic, the composite relative risk and 95% confidence interval is represented by the middle and width of the diamond, respectively. The I2 statistic was used to estimate heterogeneity across studies, with values greater than 50% is considered as substantial heterogeneity. In this meta-analysis, the investigators used a random-effects model in order to take into account the methodological variation especially with regards to dosing of trial drugs and diverse clinical definitions of acute coronary syndrome between studies.
In the event that significant heterogeneity was detected upon analysis, sub-group analysis was done to shed more light on the heterogeneity.
RESULTS
Literature Search
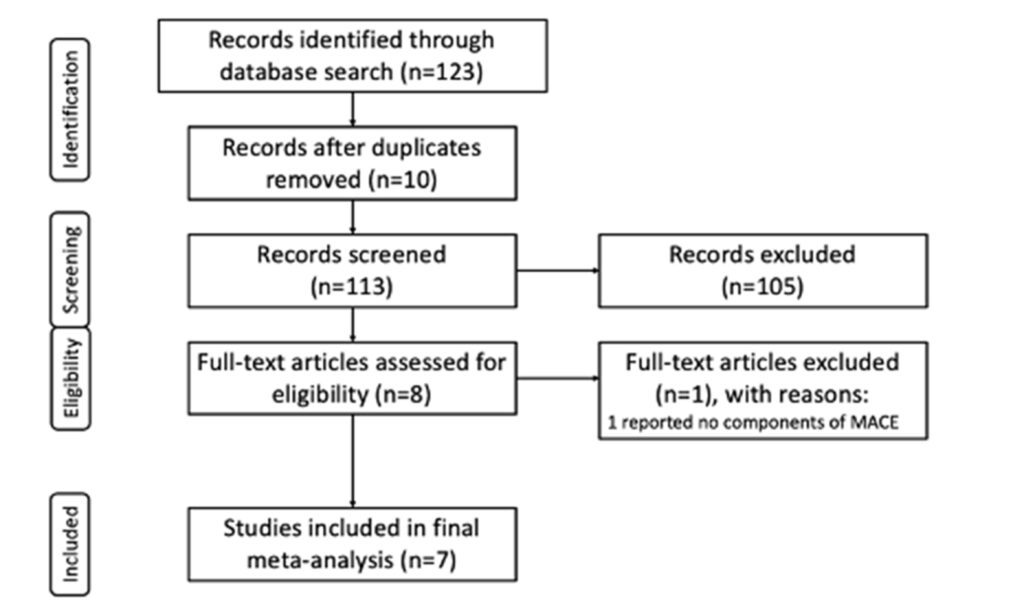
The electronic literature search yielded a total of 123 studies. After the removal of 10 duplications, the remaining studies underwent primary screening of their title and abstract. Following removal of 105 studies upon screening, eight (8) studies underwent full-text review, and seven (7) were included in the final meta-analysis based on inclusion and exclusion criteria (Figure 1)
Description of Selected Studies
A summary of the individual characteristics of the selected studies, based on each study’s population, intervention, control, and outcomes, are tabulated in Table 1.
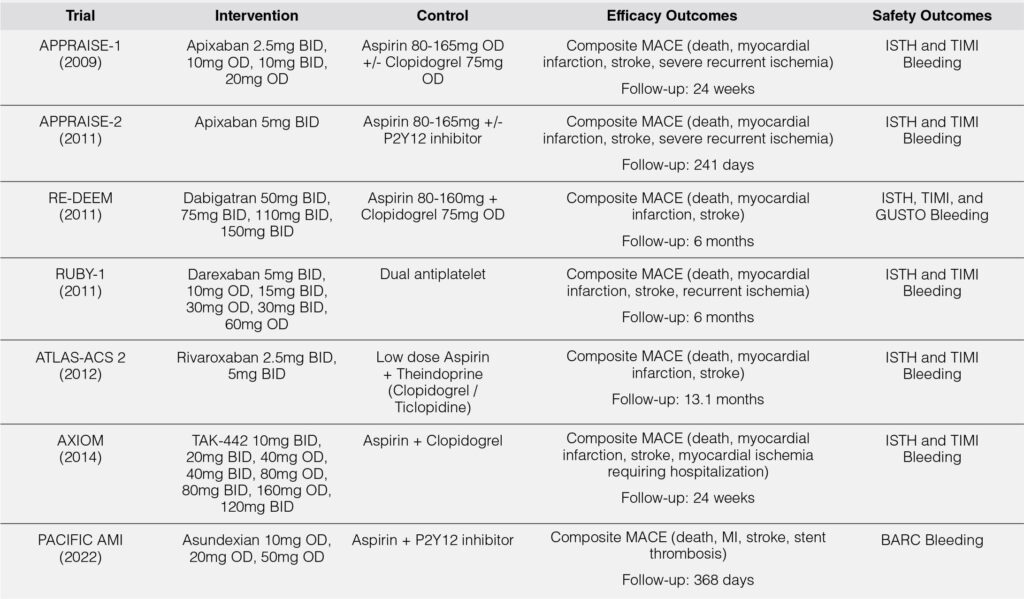
All of the studies used direct oral anticoagulants plus dual antiplatelet therapy in the intervention arm and dual antiplatelet in the control arm.
Of the DOACs available in the market, the ones used were apixaban, dabigatran, darexaban (YM-150), rivaroxaban, TAK-442, and asundexian. Of note, only apixaban, dabigatran, and rivaroxaban are the commonly used DOACs for other indications such as atrial fibrillation or venous thromboembolism. All studies had a base antiplatelet of aspirin within the range recommended as maintenance in most acute coronary syndrome guidelines. However, the included studies had a varied second antiplatelet, usually a P2Y12 inhibitor, which was commonly clopidogrel and was mostly upon the discretion of the treating physician upon enrollment of the study participants in the trial. This meta-analysis however included all studies regardless of the P2Y12 inhibitor used.
The identified studies used similar designs utilizing multiple test doses and dosing schedules of the intervention drug on top dual antiplatelet therapy and stratified as such and compared each of these stratifications to the placebo arm. Since these were the study designs used, this meta-analysis will do a two-tiered analysis wherein a meta-analysis of the total doses versus placebo will be done as well as a meta-analysis on the lowest dose versus placebo. Since previous studies have shown increased bleeding risk with triple antithrombotic therapy, the lowest dose will be analyzed as this assumes that it has the lowest risk for bleeding.
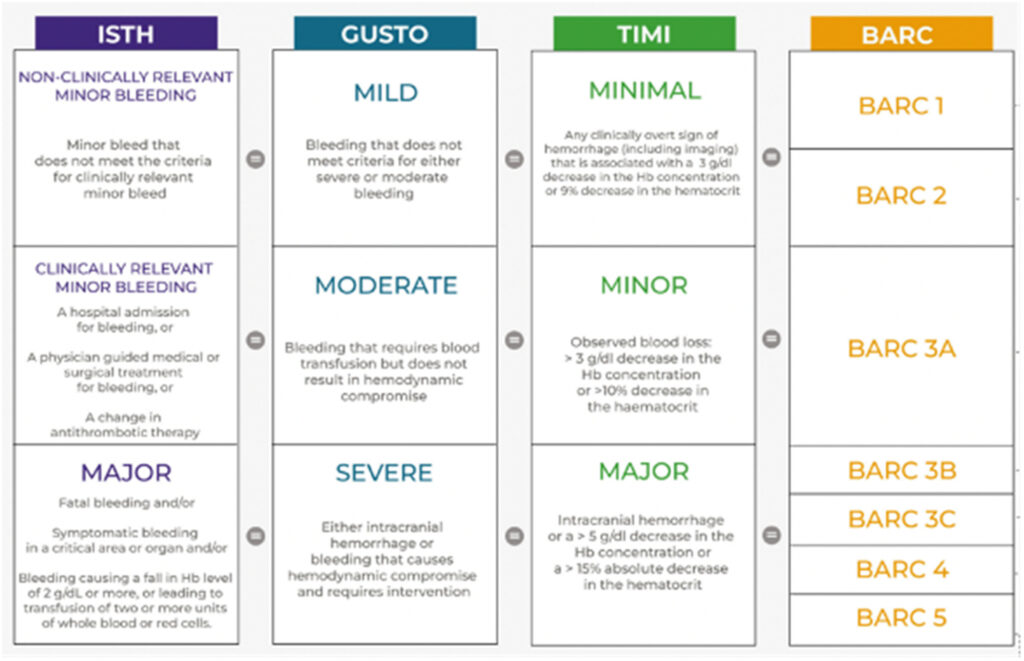
All of the studies had similar efficacy outcomes in the form of composite major adverse cardiovascular events (MACE) which included death, myocardial infarction, and stroke. In terms of safety outcomes, all included studies used the ISTH and TIMI definitions of bleeding except for the PACIFIC AMI Trial, which used the BARC definition of bleeding.
Although these definitions may have overlaps, they are still similar as to the degree, severity, and site of bleeding. A summary of this comparison can be found in Figure 2.27 Adjustments were made in the analysis to account for these nuances in the definition of bleeding.
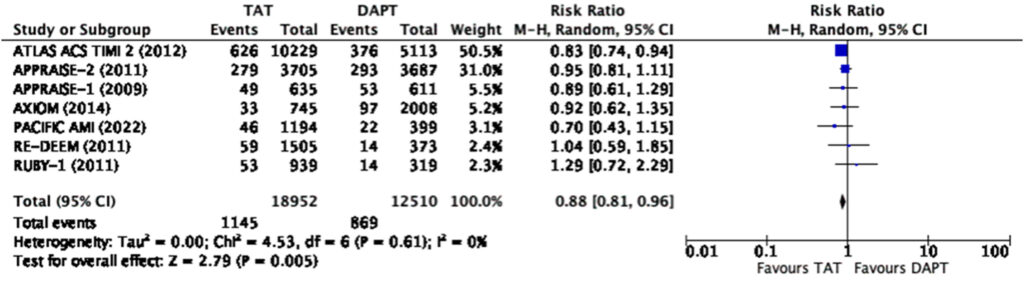
Efficacy and Safety Outcomes: Total Population
When analyzed using the total population in the individual
studies, our analysis showed that triple antithrombotic therapy
significantly reduces the risk of composite MACE (RR 0.88, 95%
CI 0.81-0.96) and myocardial infarction (RR 0.88, CI 0.78-0.98)
(Figure 3, 4).
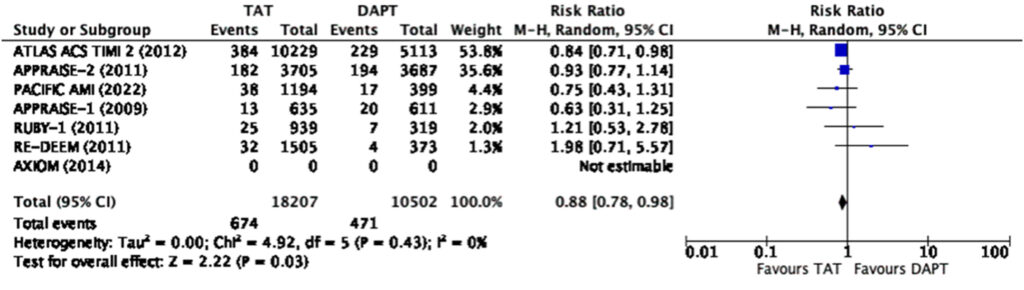
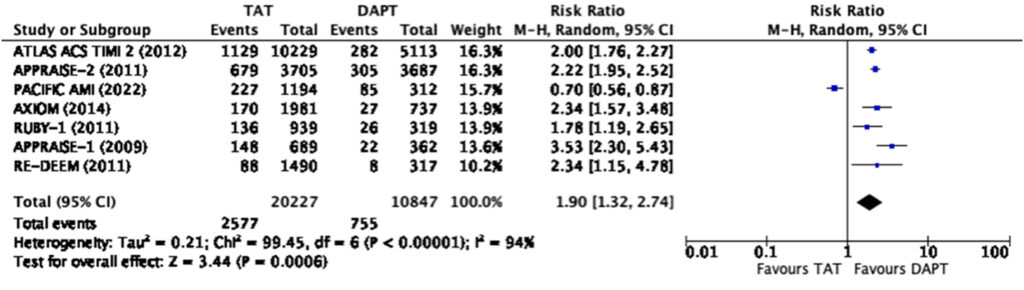
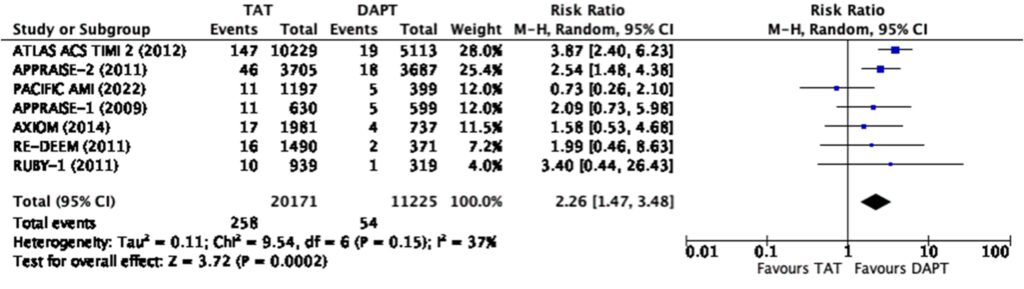
However, there was a significant risk of any bleeding (RR=1.9, 95% CI 1.32-2.74) and major bleeding (RR=2.26, 95% CI 1.47-3.48) (Figure 5, 6) among those treated with triple antithrombotic therapy as compared to those treated with dual-antiplatelet therapy alone.
Efficacy and Safety Outcomes: Lowest Dose
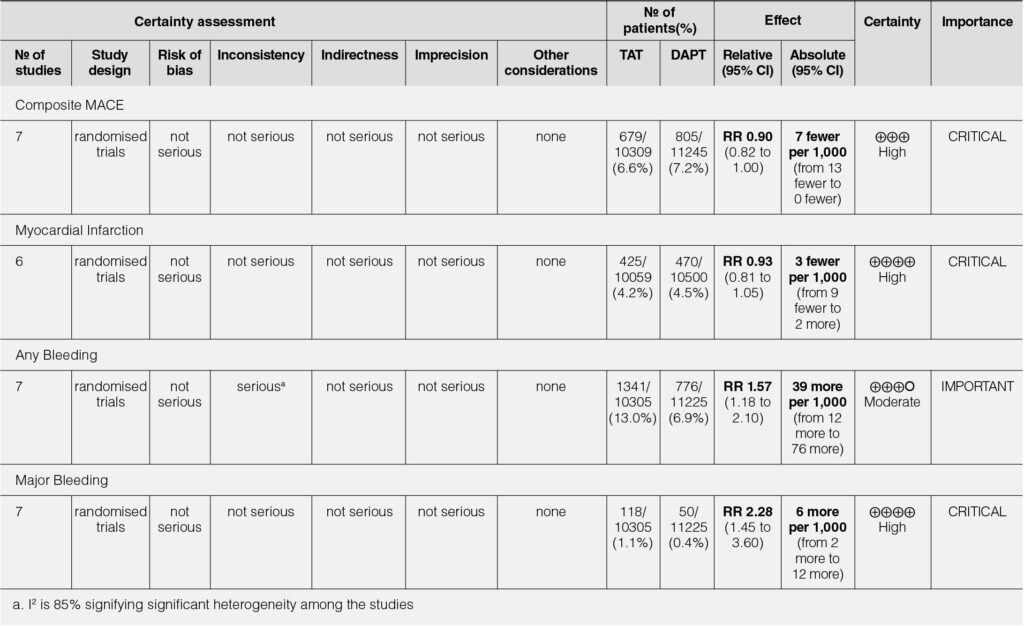
When analyzed using only the lowest dose cohort with a total sample size of 21,554, our analysis showed that triple antithrombotic therapy does not significantly reduce the risk of composite MACE (RR=0.90, 95% CI 0.82-1.00) nor does it significantly reduce the risk of myocardial infarction (RR=0.93, 95% CI 0.81-1.05) (Figure 7,8).
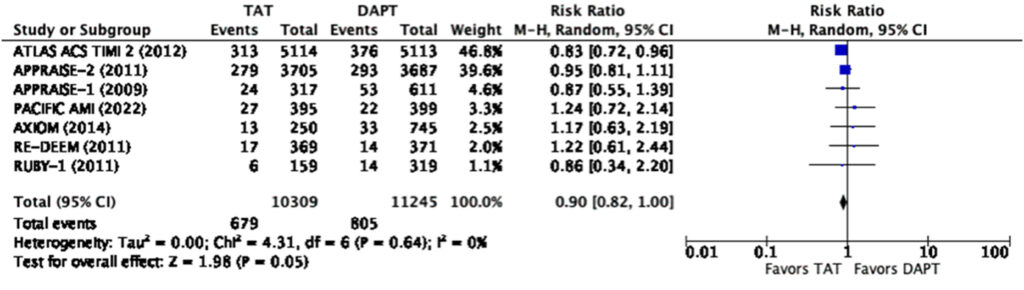
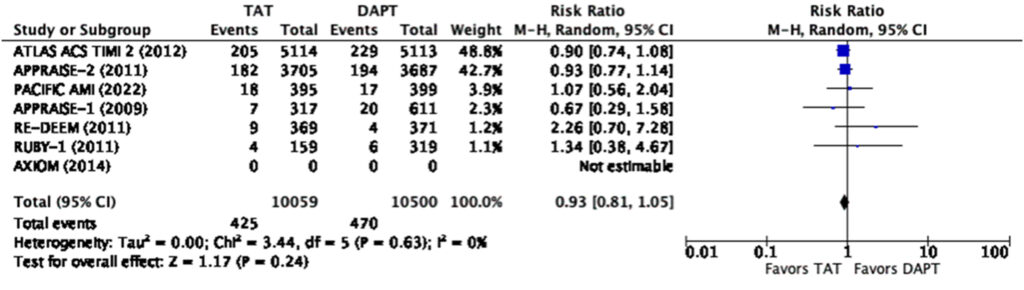
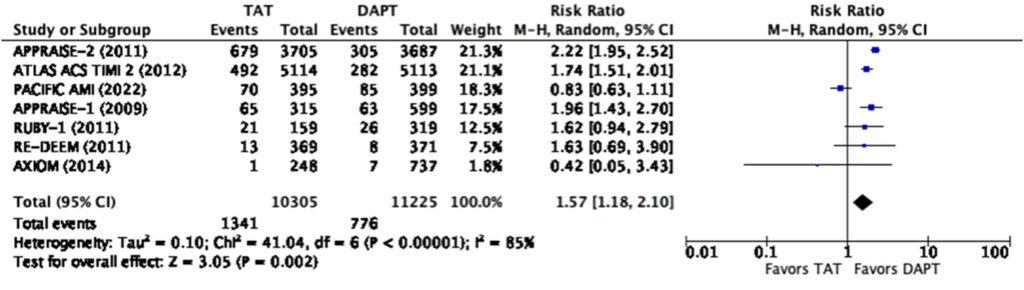
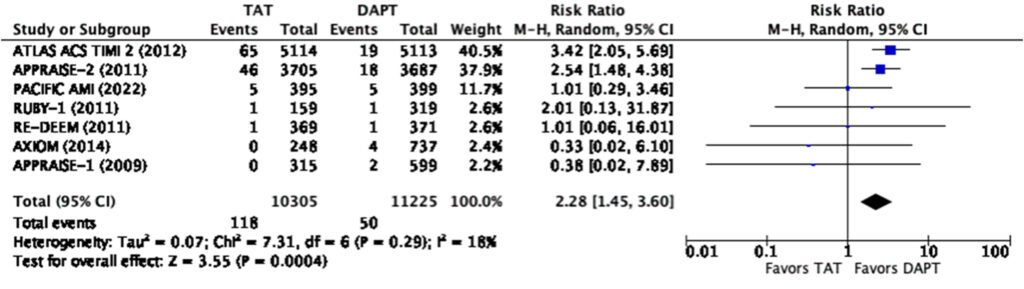
Even at the lowest dose used in the representative studies, there was a significantly increased risk for any bleeding (RR=1.57, 95% CI 1.18-2.10) and major bleeding (RR=2.28, 95% CI 1.45-3.60) (Figure 9, 10). There was a note of significant heterogeneity (i2=85%) in the analysis for any bleeding (Figure 9).
The risk of bias of the studies assessed using the Revised Cochrane Risk-of-Bias 2 tool for Randomized Trials (RoB2) showed that there was no risk of bias among all the studies included in this meta-analysis. Hence, this meta-analysis had an overall low risk of bias.
The quality of evidence was assessed using the GRADE system utilizing the GRADEPro online application. The strength of evidence was high among all outcomes except for any bleeding which showed moderate level of evidence because of the significant heterogeneity present in the analysis of this specific outcome. Therefore, it is acceptable to generalize the findings into a recommendation.
DISCUSSION
Acute coronary syndrome (ACS) remains a leading cause of morbidity and mortality. Ischemic burden remains high among patients with ACS despite being on optimal medical treatment with dual antiplatelet therapy, and there is a persistent residual risk in recurrent myocardial infarction and cardiac death among patients with recent ACS.3, 4, 5 Hence, strategies such as triple antithrombotic therapy have been suggested and studied in various multiple clinical trials. This meta-analysis aimed to analyze these studies as a whole.
Overall, this meta-analysis showed that triple antithrombotic therapy (TAT) when compared to dual-antiplatelet therapy (DAPT) had a modest reduction in composite major adverse cardiovascular events and myocardial infarction at best when analyzed cumulatively. When used at the lowest dose possible, TAT has no significant difference in terms of MACE and myocardial infarction as compared to DAPT. On top of this, regardless of the dose used and even at only the lowest dose possible, TAT had a significantly higher risk for any or major bleeding.
The findings of this meta-analysis are consistent with a previous meta-analysis done in 2013 by Oldgren et. al.23 This current study included two more trials – AXIOM in 2014 and PACIFIC AMI in 2022 – and serves as an update to the previous meta-analysis done. It also excluded another trial, the ESTEEM Trial, which only used aspirin alone in the control arm. This updated meta-analysis was pursued because in the PACIFIC AMI trial, TAT showed a significantly lower risk of any bleeding than DAPT (Figure 5) which is unusual and inconsistent with mechanistic principles and inconsistent with findings of other previous studies. The DOAC used in PACIFIC-AMI is a relatively new drug, asundexian, with its mechanism being a direct factor XIa inhibitor.28 The inhibition at this level occurs before the single common pathway of the coagulation cascade as compared to direct factor Xa or direct thrombin inhibition which acts on the common pathway already. This has been postulated as the reason why there is less bleeding with the use of this new direct oral anticoagulant.
When looking closer into the forest plot for the reduction in MACE and myocardial infarction among those in the cumulative dose analysis (Figure 3, 4), we surmise that the total effect was contributed much by the ATLAS-ACS 2 trial, a large trial on the use of rivaroxaban at a dose of 2.5mg or 5mg twice a day among patients with recent ACS on top of DAPT. The large population size of this convincing trial was the one that skewed the results towards statistical significance. We note however that at a dose of 2.5mg twice a day on top of DAPT, rivaroxaban no longer reduces the risk of myocardial infarction but still reduces the risk of composite MACE (Figure 7, 8). The rest of the individual drugs, including asundexian in the PACIFIC-AMI trial, did not show such a relationship which further strengthens the observation that the ATLAS-ACS 2 trial was the one which skewed the results as such.
Based on the pattern seen in the available trials, there is a trend showing that rivaroxaban is the most efficacious in terms of preventing recurrent ischemic events among patients with coronary artery disease. Among the DOACs, it has been proven in the AFIRE Trial that rivaroxaban alone was non-inferior when compared to rivaroxaban plus single antiplatelet therapy in preventing major adverse cardiovascular events among patients with atrial fibrillation and stable coronary artery disease with a significantly lower risk of bleeding.37 There have been no dedicated studies comparing DOACs with Aspirin for secondary prevention of CAD among those without Atrial Fibrillation.
In terms of overall bleeding risk, the addition of a direct oral anticoagulant at any dose still shows an increased risk of any and major bleeding. When scrutinizing the forest plots, we note that asundexian when added to DAPT shows a decreased risk for bleeding compared to DAPT alone as seen in the PACIFICAMI Trial. This could be due to the fact that asundexian has been shown to have a lower bleeding risk when compared to other direct oral anticoagulant. The PACIFIC-AF Phase 2 randomized trial done in 2022, compared asundexian to apixaban among patients with atrial fibrillation, and showed that there was a significantly lower risk of bleeding in the treatment arm, asundexian, compared to the control arm, apixaban, without a significant difference in the reduction of stroke.29 Based on a previous meta-analyses, apixaban was shown to have the lowest bleeding risk when compared to rivaroxaban, edoxaban, and dabigatran.30-36 Hence, asundexian, which has an even lower risk of bleeding than apixaban, shows much promise and potential to be an alternative direct oral anticoagulant in various clinical settings.
It can be noted in the analysis that there was significant heterogeneity in terms of bleeding with an I2 85% in the low dose and I2 94% in the high dose analysis respectively. It can be seen in the graphs that the PACIFIC AMI trial again was the outlier in the analysis. One of the possible reasons for this is the use of the BARC scale instead of the TIMI and ISTH scales for bleeding in this trial which may lead to some overlaps (Figure 2).
CONCLUSION
Overall, the role of antithrombotic therapy in the management of ACS and CAD among patients without atrial fibrillation is still a field which needs to be explored even further. Despite the emergence of new evidence from latest trials, collective evidence of our meta-analysis still shows an elevated risk-benefit ratio for TAT compared to DAPT in the setting of ACS without AF. Hence, further studies should likely focus on DOACS that have a better safety profile while maintaining efficacy for reducing MACE in patients with recent ACS. Weighing the bleeding risk and thrombotic risk remains to be one of the major decisions points to consider triple therapy among patient with AF with recent ACS, and the TAT strategy may be considered among patients with low bleeding risk but high thrombotic risk.
REFERENCES
1. Bergmark BA, Mathenge N, Merlini PA, et. al. Acute coronary syndromes. Lancet. 2022 2-8 April; 399(10332): 1347–1358. doi: 10.1016/S0140-6736(21)02391-6 CrossRef Pubmed
2. Philippine Statistics Authority. 2022 Causes of Deaths in the Philippines (Preliminary as of 31 October 2022). PSA Press Release. Jan 23 2023
3. Lewis BS, Mehta SR, Fox KA, et al. Benefit of clopidogrel according to timing of percutaneous coronary intervention in patients with acute coronary syndromes: further results from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Am Heart J 2005;150:1177-1184 CrossRef Pubmed
4. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-2015 CrossRef Pubmed
5. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-1057 CrossRef Pubmed
6. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016; 375:2423–2434. doi: 10.1056/NEJMoa1611594 CrossRef Pubmed
7. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, Maeng M, Merkely B, Zeymer U, Gropper S, et al; RE-DUAL PCI Steering Committee and Investigators. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017; 377:1513–1524. doi: 10.1056/
NEJMoa1708454 CrossRef Pubmed
8. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, et al; AUGUSTUS Investigators. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019; 380:1509–1524. doi: 10.1056/NEJMoa1817083 CrossRef Pubmed
9. Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, Batushkin V, Campo G, Lysak Z, Vakaliuk I, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a
randomised, open-label, phase 3b trial. Lancet. 2019; 394:1335–1343. doi: 10.1016/S0140-6736(19)31872-0 CrossRef Pubmed
10. Merlini PA, Bauer KA, Oltrona L, et al. Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation 1994;90:61-68 CrossRef Pubmed
11. Oldgren J, Wallentin L, Alexander JH, et. al. New oral anticoagulants in addition to single or dual antiplatelet therapy after an acute coronary syndrome: a systematic review and meta-analysis. Eur Heart J. 2013 Jun;34(22):1670-80. doi: 10.1093/eurheartj/eht049 CrossRef Pubmed
12. Moher D, Liberati A, Tetzlaff J et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Journal of Clinical Epidemiology Oct 2009; 62(10): 1006-1012. doi: 10.1016/j.jclinepi.2009.06.005 CrossRef Pubmed
13. Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org
14. Agoritsas T, Merglen A, Courvoisier DS, et al. Sensitivity and Predictive Value of 15 PubMed Search Strategies to Answer Clinical Questions Rated Against Full Systematic Reviews. Journal of Medical Internet Research May-Jun 2012; 14(3): e85. doi: 10.2196/jmir.2021 CrossRef Pubmed
15. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. CrossRef Pubmed
16. Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013.
17. Review Manager (RevMan) [Computer program]. Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
18. Alexander JH, Lopes RD, James S, et. al. Apixaban with Antiplatelet Therapy after Acute Coronary Syndrome. New England Journal of Medicine 2011; 365:699-708. doi: 10.1056/NEJMoa1105819 CrossRef Pubmed
19. Alexander JH, Becker RC, Bhatt DL, et. al. Apixaban, an Oral, Direct, Selective Factor Xa Inhibitor, in Combination With Antiplatelet Therapy After Acute Coronary Syndrome. Circulation. 2009; 119:2877–2885. doi: 10.1161/CIRCULATIONAHA.108.832139 CrossRef Pubmed
20. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in Patients with a Recent Acute Coronary Syndrome. New England Journal of Medicine 2012; 366:9-19. doi: 10.1056/NEJMoa1112277 CrossRef Pubmed
21. Wallentin L, Wilcox R, Weaver WD, et. al. Oral ximelagatran for secondary prophylaxis after myocardial infarction: the ESTEEM randomized controlled trial . The Lancet September 2003; 362: 789-797. doi: 10.1016/S0140- 6736(03)14287-0 CrossRef Pubmed
22. Steg PG, Mehta SR, Jukema JW, et.al. RUBY-1: a randomized, doubleblind, placebo-controlled trial of the safety and tolerability of the novel oral factor Xa inhibitor darexaban (YM-150) following acute coronary syndrome. Eur Heart J. 2011 Oct;32(20):2541-54. doi: 10.1093/eurheartj/ehr334 CrossRef Pubmed
23. Oldgren J, Budaj A, Granger C, et. al. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J. 2011 Nov; 32 (22): 2781-9. doi: 10.1093/eurheartj/ehr113. CrossRef Pubmed
24. Rao S, Kirsch B, Bhatt D, et. al. A Multicenter, Phase 2, Randomized, Placebo-Controlled, Double-Blind, Parallel-Group, Dose-Finding Trial of the Oral Factor XIa Inhibitor Asundexian to Prevent Adverse Cardiovascular Outcomes After Acute Myocardial Infarction. Circulation. 2022;146:1196–
1206. doi: 0.1161/CIRCULATIONAHA.122.061612 CrossRef Pubmed
25. Bates, E. R., Bhatt, D. L., Cao, C., Holmes, D., et.al. Phase 2 study of TAK-442, an oral factor Xa inhibitor, in patients following acute coronary syndrome. Thrombosis and Haemostasis, 111(06), 1141–1152. doi:10.1160/TH13-07-0543 CrossRef Pubmed
26. Choxi R, Kapoor K, Mackman N, and Jovin I. Direct Oral Anticoagulants and Coronary Artery Disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2022;42:553–564. doi: 10.1161/ATVBAHA.121.317171 CrossRef Pubmed
27. Galli M, Laborante R, Andreotti F, et. al. Bleeding Complications in Patients Undergoing Percutaneous Coronary Intervention. Rev. Cardiovasc. Med. 2022; 23(8): 286 doi: 10.31083/j.rcm2308286 CrossRef Pubmed
28. Heitmeier S, Visser M, Tersteegen A, Dietze-Torres J, Glunz J, Gerdes C, Laux V, Stampfuss J, Roehrig S. Pharmacological profile of asundexian, a novel, orally bioavailable inhibitor of factor XIa. J Thromb Haemost. 2022 Jun;20(6):1400-1411. doi: 10.1111/jth.15700 CrossRef Pubmed
29. Piccini JP, Caso V, Connolly SJ, et. al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): a multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. The Lancet 2022 Apr; 399(10333):1383-1390 CrossRef Pubmed
30. Sherrill B, Fernandez M, Wang J et al. Network Meta-analysis of relative efficacy and safety of edoxaban versus other novel oral anticoagulants (NOACs) among atrial fibrillation with CHADS2 score >2. Journal of American College of Cardiology, 2015; 65(10). doi: 10.1016/S0735-1097(15)60346-1
31. Skjoth F, Larsen TB, Rasmussen LH, Lip GYH. Efficacy and safety of edoxaban in comparison with dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation. An indirect comparison analysis. Journal of Thrombosis and Hemostasis, 2014; 111(5). doi: 10.1160/TH14-
02-0118 CrossRef Pubmed
32. Hill NR, Sandler B, Bergrath E et al. A Systematic Review of Network Meta-Analyses and Real-World Evidence Comparing Apixaban and Rivaroxaban in Nonvalvular Atrial Fibrillation. Journal of Clinical and Applied Thrombosis/Hemostasis, 2020; 26. doi: 10.1177/1076029619898764 CrossRef Pubmed
33. Douros A, Durand M, Doyle CM et al. Comparative Effectiveness and Safety of Direct Oral Anticoagulants in Patients with Atrial Fibrillation: A Systematic Review and Meta-Analysis of Observational Studies. Journal of Drug Safety, Oct 2019; 42(10): 1135-1148. doi: 10.1007/s40264-019-00842-1. CrossRef Pubmed
34. Rutherford OC, Jonasson C, Ghanima W et al. Comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in atrial fibrillation: a nationwide cohort study. European Heart Journal – Cardiovascular Pharmacotherapy, Apr 2020; 6(2): 75-85. doi: 10.1093/ehjcvp/pvz086 CrossRef Pubmed
35. Aryal MR, Gosain R, Donato A, et al. Systematic review and meta-analysis of the efficacy and safety of apixaban compared to rivaroxaban in acute VTE in the real world. Journal of blood advances, Jun 2019; 3(15): 2381-2387. 10.1182/ bloodadvances.2019000572 CrossRef Pubmed
36. Lee SR, Choi EK, Han KD et al. Comparison of Once-Daily Administration of Edoxaban and Rivaroxaban in Asian Patients with Atrial Fibrillation. Scientific Reports, 2019;9:6690. doi: 10.1038/s41598-019-43224-4 CrossRef Pubmed
37. Yasuda S, Kaikita K, Akao M, et al. Antithrombotic Therapy for Atrial Fibrillation with Stable Coronary Disease (AFIRE Trial). N Engl J Med 2019; 381:1103-1113. doi: 10.1056/NEJMoa1904143 CrossRef Pubmed
Copyright Information
