Case Report
August 2025, 34:2
First online: 18 August 2025
Case Report
Fulminant Myocarditis in a Filipino Female with Hormonal Receptor Discordant Primary Breast Cancer with Bone Metastases: Fatal Immune-related Adverse Event
Renato C. Ong, Jr., RMT, M.D., FPCP, FPCC,1 Maria Kristina Cecilia P. Ozaeta-Lorilla, MD, FPCP, FPCC,1 Ma. Belen E. Tamayo, MD, FPCP, FPSMO,1 Ramon D. Francisco, MD, FPCP1
Main Author: Renato C. Ong Jr
Orcid ID No: 0000-0002-0433-7511
Funding: None.
Disclosures: None.
Main Author’s Contact Details:
Address for correspondence: Renato C. Ong Jr., MD. Section of Cardiology Makati Medical Center 6FT2 2 Amorsolo Street, Legaspi Village, Brgy. San Lorenzo, Makati City, Philippines 1229.
Mobile Telephone No. +6388888999.
Email: r.ongjrmd@gmail.com
ABSTRACT
BACKGROUNDAlthough immune-checkpoint inhibitor-related myocarditis is uncommon,1-5 it is potentially fatal with a steep mortality of 25% to 50% compared with other immune-related adverse events.
CASE REPORTA 63-year-old hypertensive, diabetic Filipino female known to have triple-negative breast cancer with bone metastases came in because of dyspnea and desaturation. In November 2005, the patient was diagnosed with biopsy-confirmed invasive ductal carcinoma, estrogen- (ER) and progesterone receptor (PR) positive, HER2/neu negative. Docetaxel and epirubicin with radiation therapy (RT) were administered thereafter, she was maintained on anastrozole for five years, then she underwent remission. Nine years later, tumoral spread to the frontal bone, T5 vertebra and left adrenal gland was noted and biopsy revealed triple-hormone-negative mucinous carcinoma favoring metastasis. Genetic sequencing exhibited high tumor mutational burden, pembrolizumab was started. She tolerated the treatment until days after cycle 10, she developed dyspnea at rest, anterior chest discomfort and desaturation. TTE showed segmental hypokinesis with preserved ejection fraction with decreased GLS. Hypotension soon ensued, necessitating vasopressors. Repeat laboratories showed increasing hsTnI, NT-proBNP and D-dimer. ECG demonstrated a new bifascicular block with prolonged QTcF. Satisfying the clinical criteria for fulminant ICI-related myocarditis, hydrocortisone was shifted to methylprednisolone. CMR confirmed prior myocarditis. After three days of methylprednisolone, oral prednisone was started and guideline-directed medical therapy for heart failure was slowly resumed. The patient was eventually discharged improved.
LEARNING POINTSIt is imperative to recognize that while immunotherapy represents a significant advancement in oncologic treatment—offering improved survival rates and enhanced quality of life for patients—it is not without risk. Rare yet potentially fatal complications may arise, necessitating vigilant monitoring and a high index of suspicion for immune-related adverse events.
INTRODUCTION
Immune checkpoint inhibitors (ICI) have revolutionized cancer therapy and have been regarded as the “magic bullet” for combating cancer.6-8 Complete response have been observed in a minority of patients and most patients develop immunerelated adverse events (irAEs) (i.e., diarrhea, colitis, rash, hypothyroidism, adrenal insufficiency).6, 8 Although with a reported incidence of only <1.2%,1-5 ICI-related myocarditis is potentially fatal with a mortality rate ranging from 25% to 50% compared with other irAEs.
CASE REPORT
A 63-year-old Filipino female known to have hypertension, diabetes, hypothyroidism and triple-negative breast cancer with bone metastases came in because of sudden onset dyspnea with associated bouts of desaturation days after completing cycle 10 of pembrolizumab.
In November 2005, the patient noted a lump on her left breast, nonmobile, 3 cm located in the upper outer quadrant for a year which was diagnosed breast cancer (BRCA), left T2N2M0. The following year, she underwent lumpectomy with frozen section, and with initial biopsy of invasive ductal carcinoma, subsequent modified radical mastectomy with axillary lymph node dissection was carried out.
Biopsy was consistent with invasive ductal carcinoma, metastasis to 4/14 axillary lymph nodes, positive for dermal invasion, areolar region and axillary fat, and for lymphovascular invasion with a pathologic diagnosis of stage IIIA (pT2pN2apMx). Immunohistochemistry breast panel assay revealed an estrogen receptor (ER) and progesterone receptor (PR) positive with no gene amplification for HER-2/neu by fluorescence in situ hybridization (HER 2/neu:chromosome 17 ratio of 1.1).
Six cycles of adjuvant chemotherapy with docetaxel [cumulative dose (CD) of ~600 mg/m2] and epirubicin (CD, ~720 mg/m2 equivalent to CD, ~440 mg/m2 doxorubicin), followed by intensity modulated radiation therapy (IMRT) to the left chest ~40 Gy divided in 33 fractions [mean heart dose (MHD), ~4-10 Gy]. Thereafter, she was maintained on oral anastrozole.
On follow up, there was lymphedema in the left upper extremity. Duplex scan demonstrated deep venous insufficiency of the bilateral axillary and brachial veins where manual lymph drainage and bandaging was done. Although with normal breast on physical exam, breast ultrasound otherwise revealed a complex well-defined mass with cystic component in the mid outer breast region along the midclavicular line, Breast Imaging Reporting and Data System (BIRADS) category 5 (highly suggestive of malignancy). Chest HRCT ruled in tumor recurrence in the mastectomy site infiltrating the underlying pectoralis muscle and overlying subcutaneous fat pad. Anastrozole was continued under close monitoring.
In 2009, she remained asymptomatic with stable disease and underwent left breast reconstruction surgery. Follow-up SPECT studies and mammograms showed neither suspicious findings nor evidence of bone metastatic disease. Anastrozole was completed for five years, and thereafter the patient went into remission.
Nine years later, mass on the right forehead was noted; possible tumor recurrence was considered. CT scan examination revealed the following: head – lytic changes in the calvarium with an expansile soft tissue component in the right frontal bone suggestive of osseous metastasis with note of a new slightly hypodense focus with no enhancement in the left inferior parietal lobule superior to the temporal lobe; chest – mediastinal and right hilar lymphadenopathy with mixed lytic-sclerotic lesions in T5 vertebra, both likely metastatic; and abdomen -heterogeneously enhancing isodense nodule in the left adrenal gland, likely metastatic.
The patient then underwent mediastinoscopy, biopsy of the anterior mediastinal lymph nodes revealing mucinous carcinoma favoring metastasis. Immunohistochemical staining demonstrated triple (ER/PR/Her2/neu) negative and PIK3CA-mutation positive breast cancer. Nine cycles of eribulin were delivered. The patient had concomitant IMRT to the spine [~45 Gy in 20 fractions (MHD, 0 Gy)], and another directed to the frontal mass [~30 Gy in 10 fractions (MHD, 0 Gy)]. Comprehensive genetic profile sequencing showed a high tumor mutational burden (35.1 mutations/Mb) with sensitivity to immune-checkpoint inhibitors (ICI), hence pembrolizumab immunotherapy was started. ~6 days post cycle 10 pembrolizumab, the patient developed dyspnea on exertion necessitating as needed home oxygen supplementation and bedrest. On the day of admission, the patient strained while defecating and developed dyspnea at rest with associated anterior chest discomfort and desaturation to as low as 65%. Oxygen supplementation was increased, and the patient was brought to the Emergency Department (ED) for evaluation.
At the ED, the patient was normotensive, although tachycardic and tachypneic. There was palpable mass at the right frontal area with pale conjunctivae, nondistended neck veins with bilateral basal rales. Chest findings showed regular rhythm with distinct S1 and S2 with no S3. There was no bipedal pitting edema with full equal pulses. Diagnostics showed a decrease in hematocrit (22.7%), platelets (85,000 cells/μL) and cortisol (1.86 μg/dL); and an increase in: NT-proBNP (455 pg/mL), D-dimer (3.72 μg/mL), PTH (119.48 pg/mL), ferritin (2,201 ng/mL) and LDH (324.54 U/L). Whereas the rest of the analytes including thyroid panel, anti-TPO, creatinine, electrolytes, procalcitonin and hsTnI were normal. ECG showed sinus tachycardia with complete right bundle branch block (Figure 1). Transthoracic echocardiography (TTE) showed hypokinesis of the anterior, anterolateral, inferior and inferolateral walls from base to apex with preserved LVEF of 61% and moderate tricuspid regurgitation. There was a ~42.7% relative decrease in average left ventricular global longitudinal strain (LV-GLS) from (-) 19.2% to (-) 11%. She had negative DVT screening of the lower extremities. Diuretics, low dose angiotensin-receptor blocker, trimetazidine and statin were started. And the patient was likewise scheduled for cardiac magnetic resonance (CMR).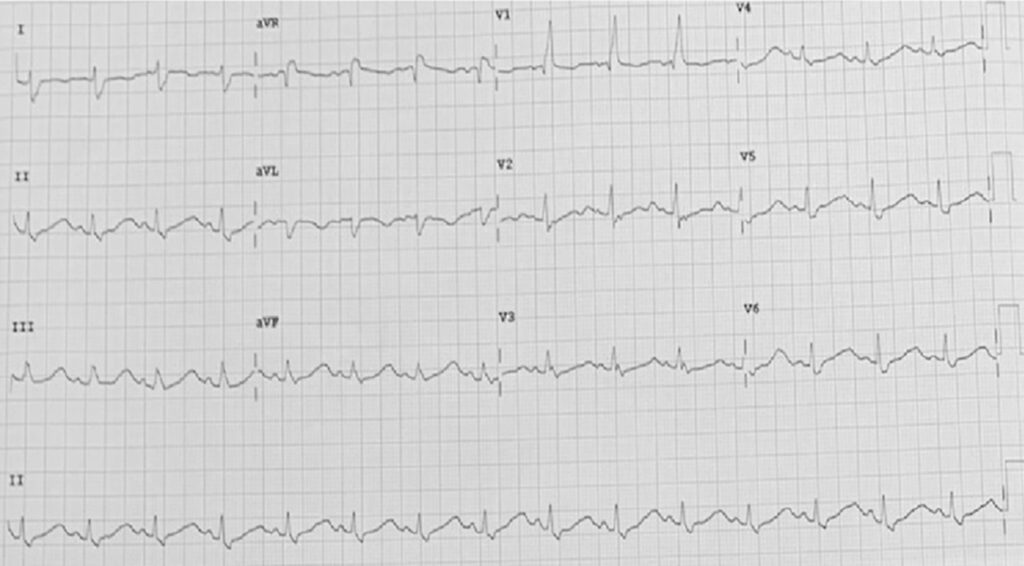
Along the course in the wards, she developed intermittent anterior chest discomfort and palpitations with associated dyspnea at rest. Repeat ECG showed new bifascicular block, premature atrial contractions (PAC) in bigeminy, nonspecific ST-T wave changes and normal QTcF. Initially, vital signs remained stable; however hypotension soon ensued with a BP to as low as 60/40 mmHg which was refractory to fluid resuscitation, necessitating two vasopressors (norepinephrine and vasopressin). All BP medications with diuretics were discontinued. Mean arterial pressure (MAP) above 60 mmHg was maintained, and she was transferred to the ICU for closer monitoring. Hydrocortisone was likewise started to cover for relative adrenal insufficiency.
Repeat diagnostics showed interval increase in: hsTnI (0.681 from 0.003 ng/mL), NT-proBNP (654.9 from 455 pg/mL), D-dimer (9,875 ng/mL FEU), and procalcitonin (0.36 from 0.22 ng/mL). Repeat ECG demonstrated no significant change from previous aside from resolution of the PACs and prolonged QTcF of 628 msec, whereas repeat TTE showed the same hypokinetic regions with preserved LVEF of 60% and beaking motion of the interventricular septum. She had episodes of atrial tachycardia, frequent runs of PVCs and PACs on the cardiac monitor. Electrolytes were maintained to optimum levels.
Initially, there was a plan for referral to interventional cardiology for coronary angiogram possible angioplasty, however, was placed on hold because the patient’s profile and repeat cardiac biomarkers satisfied the clinical diagnostic criteria for fulminant ICI-related myocarditis from pembrolizumab. Hydrocortisone was shifted to methylprednisolone 1 gm IV every 24 hours for 3 days. Also, broad spectrum antibiotics were likewise started to cover for possible sepsis.
As the vasopressors were titrated down, the patient developed intermittent bouts of shortness of breath with associated desaturations (sPO2min 80%) and bradycardia (CRmin 38 bpm). The patient’s risk to develop venous thromboembolism was high, but her risk for bleeding was also high, so low dose enoxaparin was initiated for prophylaxis.
There was an interval increase in NT-proBNP (21,726.03 from 654.9 pg/mL) with an increased hsCRp (4.36 mg/dL) on repeat work up, whereas an interval decrease in the following was noted: D-dimer (6,970 from 9,875 ng/mL FEU) and hsTni (0.063 from 0.681 ng/mL). Bone marrow aspiration biopsy showed a markedly hypocellular bone marrow (<5%) with metastatic poorly differentiated adenocarcinoma. The tissue was sent for breast panel which was negative for the three analytes: no nuclear staining for ER and PR, and a faint, incomplete membranous expression in 10% of the invasive tumor cells for HEr2/neu.
The CMR showed top normal LV size with preserved systolic function with hypokinetic anterior segments, basal inferolateral, basal anterolateral and apical lateral segments; paradoxical septal wall motion; no myocardial edema; linear mid-wall enhancement (non-ischemic pattern) involving the septum and basal inferolateral segment; patchy mid wall enhancement (non-ischemic pattern) involving the basal to mid anterior, anterolateral and inferior, and mid inferolateral segments suggestive of prior myocarditis. There were patchy and confluent signal abnormalities involving the lungs bilaterally with small bilateral pleural effusion, pulmonary edema was considered. Diuresis was slowly resumed as allowed by the BP (Figure 2).
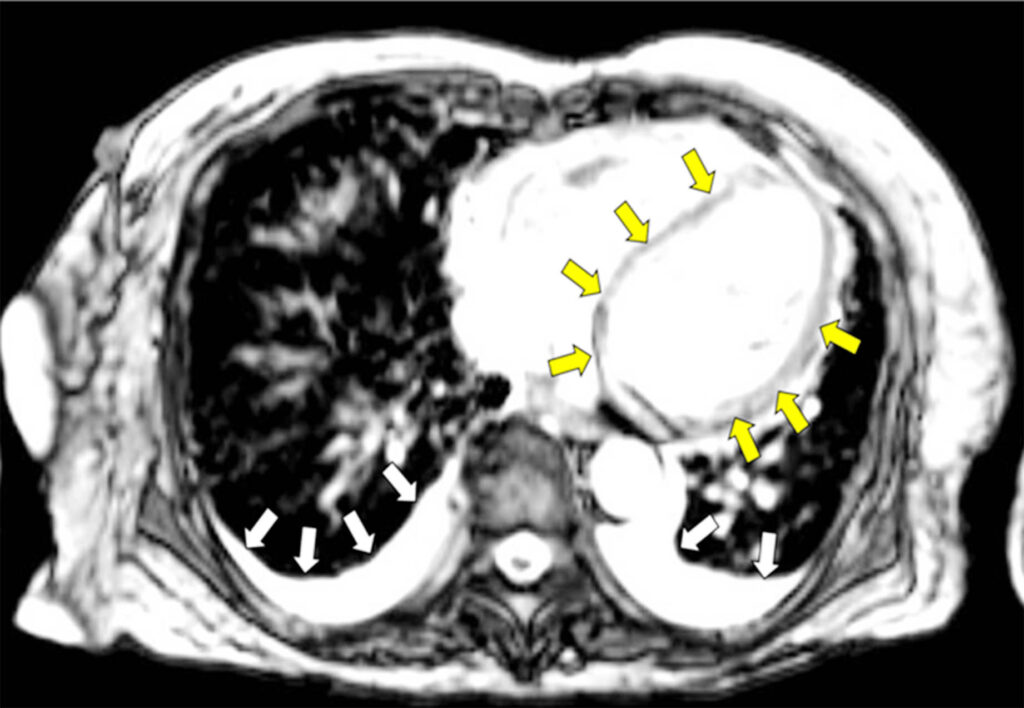
After 3 days of methylprednisolone, MAP stabilized, and vasopressors were eventually put off. Oral prednisone was started at 1 mg/kg/day with weekly tapering of doses. Guideline-directed medical therapy for heart failure [angiotensin-receptor/neprilysin inhibitor (ARNI), mineralocorticoid receptor antagonist (MRA), sodium-glucose cotransporter-2 Inhibitors (SGLT2Is) and beta blocker) were slowly initiated with close VS monitoring.
The patient was eventually discharged improved. A multidisciplinary team meeting discussed the plans for the patient, with the background of an aggressive disease with high tumor mutational burden, a chemotherapeutic regimen with palliative intent was to be planned out to be given upon myocardial recovery and stabilization.
DISCUSSION
Immune checkpoint inhibitors (ICIs) are monoclonal antibodies that target the regulation receptors in the immune system such as the programmed cell death ligand 1 (PD-L1), programmed cell death receptor 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).12 They enhance the immune system and prevent immune invasion. However, non-specific T-cell de-repression can cause a variety of immune related adverse events including gastrointestinal, endocrine, dermatologic with having a smaller proportion having potential for fatal outcomes such as neurotoxicity, pulmonary toxicity, and cardiotoxicity.13
Our patient was given Pembrolizumab, a PD-1 inhibitor, for recurrent, metastatic triple-negative breast cancer with high tumor mutational burden-high cancer. After cycle 10 of her chemotherapy, she then developed dyspnea, desaturation, anterior chest discomfort with abnormalities in ECG and TTE. When hypotension developed, myocarditis was suspected.
The side effects of ICIs may lead to complications such as fulminant myocarditis, myopericarditis, arrhythmias, myocardial infarction, and vasculitis. Patients at risk for higher risk of toxicity include dual ICI therapy, combination ICI therapy with other cardiotoxic therapies, patients with ICI-related non-cardiovascular events, and prior cancer therapy-related cardiac dysfunction or cardiovascular disease. According to the 2022 European Society of Cardiology Guidelines on cardio-oncology, all patients receiving ICI treatment should have an electrocardiogram and troponin assay at baseline. Patients with baseline stratification of high risk should have a TTE prior to treatment. Monitoring is challenging due to lack of evidence-based recommendation but once started, ECG, Troponin and Natriuretic peptides should be checked.14
Myocarditis is a serious complication of ICI with a high fatality rate frequently occurring during the first 12 weeks of treatment though late cases (after 20 weeks) has also been documented.15 The exact mechanism for it to occur is still uncertain. Suggested mechanisms include a shared antigen between the tumor and myocardium, T-cell receptor targeting a different but homologous muscle antigen as the tumor antigen, or certain T-cell receptors targeting dissimilar antigens.16 The first two mechanisms is similar to the proposed mechanism of viral myocarditis where the heart is targeted by a process of molecular mimicry.17 In patients who develop cardiac symptoms, new ECG abnormalities, and new increase in cardiac biomarkers, prompt evaluation with TTE is recommended and cardiac magnetic resonance (CMR) imaging be instituted when myocarditis is suspected.14 Treatment consists of the treatment termination, administration of high doses of corticosteroids as first-line therapy, and second-line immunosuppressive therapy to be initiated if patient does not improve after the 3 days of steroids.18
In our patient, methylprednisolone was given and CMR confirmed the presence of myocarditis. She was weaned from norepinephrine with further improvement of symptoms. Oral prednisone and guideline-directed medical therapy for heart failure was started. She was eventually discharged stable.
Learning point
Immunotherapy represents a significant breakthrough in oncological treatment, offering enhanced survival outcomes and improved quality of life for select patient populations. Nevertheless, this modality is not without its risks, as it can be accompanied by rare yet potentially severe adverse effects. Vigilant monitoring is essential to promptly detect and manage immune-related complications. KeywordsPembrolizumab, immune-related adverse event, IRAE, immunotherapy, heart failure, myocarditis.
REFERENCES
1. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA, Anders RA, Sosman JA, Moslehi JJ. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016; 375:1749–1755 CrossRef Pubmed
2. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018; 71:1755–1764. CrossRef Pubmed
3. Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018; 391:933. CrossRef Pubmed
4. Salem JE, Manouchehri A, Moey M, Lebrun-Vignes B, Bastarache L, Pariente A, Gobert A, Spano JP, Balko JM, Bonaca MP, Roden DM, Johnson DB, Moslehi JJ. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. CrossRef Pubmed
5. Al-Kindi SG, Oliveira GH. Reporting of immune checkpoint inhibitor-associated myocarditis. Lancet. 2018; 392:382–383. CrossRef Pubmed
6. Monfort A, Dufao C, Colacios C, et al. Anti-TNF, a magic bullet in cancer immunotherapy? J Immunother Cancer. 2019; 7: 303. doi: 10.1186/s40425-019-0802-y CrossRef Pubmed
7. Miazek-Zapala N, Slusarcyzk A, Kusowska A, et al. The “Magic Bullet” Is Here? Cell-Based Immunotherapies for Hematological Malignancies in the Twilight of the Chemotherapy Era. Cells. 2021 Jun; 10(6): 1511. doi: 10.3390/cells10061511 CrossRef Pubmed
8. El Osta B, Hu F, Sadek R, et al. A meta-analysis of immune-related adverse events (irAE) of immune checkpoint inhibitors (ICI) from cancer clinical trials. Annals of Oncology. 2016. 27(6):VI369. doi:https://doi.org/10.1093/annonc/mdw378.31 CrossRef
9. Reck M., Rodríguez-Abreu D., Robinson A., Hui R., Csöszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019;37:537–546. CrossRef Pubmed
10. Hodi F.S., Chiarion-Sileni V., Gonzalez R., Grob J., Rutkowski P., Cowey C.L., Lao C.D., Schadendorf D., Wagstaff J., Dummer R., et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492. CrossRef Pubmed
11. Albandar H, Fuqua J, Albandar J, et al. Immune-Related Adverse Events (irAE) in Cancer Immune Checkpoint Inhibitors (ICI) and Survival Outcomes Correlation: To Rechallenge or Not? Cancers (Basel). 2021 Mar; 13(5): 989. doi: 10.3390/cancers13050989 CrossRef Pubmed
12. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune Checkpoint Inhibitor Myocarditis: Pathophysiological Characteristics, Diagnosis, and Treatment. J Am Heart Assoc. 2020 Jan 21;9(2):e013757. doi: 10.1161/JAHA.119.013757. Epub 2020 Jan 21. PMID: 31960755; PMCID: PMC7033840. CrossRef Pubmed
13. Jiménez-Alejandre R, Ruiz-Fernández I, Martín P. Pathophysiology of Immune Checkpoint Inhibitor-Induced Myocarditis. Cancers (Basel). 2022 Sep 16;14(18):4494. doi: 10.3390/cancers14184494. PMID: 36139654; PMCID: PMC9497311. CrossRef Pubmed
14. Lyon AR, López-Fernández T, et al. ESC Scientific Document Group, 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardiooncology of the European Society of Cardiology (ESC), European Heart Journal, Volume 43, Issue 41, 1 November 2022, Pages 4229–4361, https://doi.org/10.1093/eurheartj/ehac244 CrossRef Pubmed
15. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer 2021;9:e002435. CrossRef Pubmed
16. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA, Anders RA, Sosman JA, Moslehi JJ. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016; 375:1749–1755. CrossRef Pubmed
17. Liu Peter P, Mason Jay W. Advances in understanding of myocarditis. Circulation. 2001;104; 1076-1082. CrossRef Pubmed
18. Thuny F, Alexandre J, Salem JE, Mirabel M, Dolladille C, Cohen-Solal A, Cohen A, Ederhy S, Cautela J; French Working Group of Cardio-Oncology. Management of Immune Checkpoint Inhibitor-Induced Myocarditis: The French Working Group’s Plea for a Pragmatic Approach. JACC CardioOncol. 2021 Jan 12;3(1):157-161. doi: 10.1016/j.jaccao.2020.12.001. PMID: 34396318; PMCID: PMC8352226. CrossRef Pubmed
Copyright Information
