Original Article
November 2024, 33:3
First online: 14 December 2024
Original Article
Emergence of Computed Tomography Angiography in Evaluation and Management of Diverse Coronary Artery Anomalies: A Single Center Registry
Abhishek Raval, MD, DM,1 Jayesh Rawal, MD, DM,2 Jayesh Prajapati, MD, DM,3 Yashpal Rana, MD,4 Hasit Joshi, MD, DM,5 Tarun Madan, MD, DNB 6
2 Professor and the Head of Department of Cardiology; SBKS Medical Institute and Research Center, Pipariya, Gujarat, India.
3 Consulting and Interventional Cardiologist. U.N. Mehta Institute of Cardiology and Research Centre (UNMICRC), Ahmedabad, India.
4 Radiologist, U.N. Mehta Institute of Cardiology and Research Centre (UNMICRC), Ahmedabad, India.
5 Consulting and Interventional Cardiologist. U.N. Mehta Institute of Cardiology and Research Centre (UNMICRC), Ahmedabad, India.
6 Consulting and Interventional Cardiologist. U.N. Mehta Institute of Cardiology and Research Centre (UNMICRC), Ahmedabad, India.
Source of support: U. N. Mehta Institute of Cardiology and Research Centre (affiliated to the B.J. Medical College, Ahmedabad, India) There were no conflict of interest and no relationship with industry.
Generative AI and AI-assisted technologies were not used in the writing process. Funding source statement: This research did not receive any specific grant from funding agencies in the public, commercial, or non-for-profit sectors.
ABSTRACT
BACKGROUNDThis registry compared the findings of different modalities in patients showing Coronary Artery Anomalies (CAA) to determine the usefulness of Multi-detector Computed Tomography Angiography (MDCTA).
METHODSA retrospective review of patients who underwent coronary imaging was performed. Patients diagnosed for CAA were included. Their results on imaging modalities were compared.
RESULTSCAAs were detected in 257 out of 17,245 patients (1.49%). Fiftyfive (0.319%) had separate origins of left anterior descending and circumflex arteries from left-sinus. ICA and MDCTA were equivalent (p=1) for its detection. Thirty-four patients had abnormal high origin near same sinus. ICA had lower sensitivity (52.9%) than MDCTA (100%) (p=0.0001) for its detection. Both modalities had 100% specificity. Anomalous coronary arteries from opposite sinus (ACAOS) were found in 88 patients (0.51%). ICA has 95.9% sensitivity, 100% specificity for diagnosis and 91.8% sensitivity for course-delineation. MDCTA has 100% sensitivity and specificity. For ACAOS, diagnostic accuracies of both were equivalent (p=0.49). Course-delineation was better with MDCTA than with ICA (p=0.05). Radiation-exposure with ICA (7.3±2mSv) was lower than that with MDCTA (14.5±3mSv) (p<0.0001). Radiation-exposure for ACAOS was more than that for other anomalies with ICA (p<0.001), but not with MDCTA (p=0.18). Radiation-exposure with ICA correlated with CAA-score (r=0.3), especially for origin and course anomalies (r=0.6). With MDCTA, that did not correlate with CAA-score (r=-0.019). Contrast-expenditure during ICA (65.55±19.9ml) and MDCTA (63.15±15.6ml) were equivalent (p=0.52). Contrast-expenditure during ICA in adults correlated with CAA-score (r=0.42), in contrast to MDCTA (r=-0.04).
CONCLUSIONMDCTA is a good modality for detection and course delineation of CAAs. Radiation and contrast-exposure with MDCTA does not correlate with complexity of CAA.
INTRODUCTION
Coronary artery anomalies (CAA) are diverse congenital anomalies. They have a prevalence ranging from 0.17% in autopsy series to 1.2% in angiography series.1 Clinical presentations may range from asymptomatic to nonfatal or even fatal myocardial infarction or arrhythmias. These malignant manifestations are more likely in patients with Anomalous Coronary Artery from the Opposite Sinus (ACAOS) especially among young athletes.1 Sometimes, the anomalous vessel, traversing between the aorta and the main pulmonary artery, may lead to sudden cardiac death.1 These anomalies are conventionally evaluated with invasive coronary angiography (ICA), as a gold-standard.1 However, due to its two-dimensional projections and absence of other soft tissue characterisation, the visualisation of a complex three-dimensional vessel course and clarification of the exact relationship with surrounding structures may be difficult, and misinterpretation is reported in up to 50% of cases.2 Echocardiography, a non-invasive tool for detection of CAAs especially in children, has a limited usefulness in adults. Computed tomography (CT) angiography offers excellent spatial resolution and identifies most of the CAAs.1, 3 Multi-detector CT angiography (MDCTA), has increased importance in the area of cardiac imaging.1 Apart from non-invasiveness, it has several advantages in coronary imaging like excellent spatial resolution demonstrating precise origin and course in relation to the surrounding structures, helping characterization of CAAs compared to ICA.1, 3 There are a few studies which compare the individual anomaly-wise results of MDCTA with ICA. We are presenting a registry of coronary evaluations at single center with specific emphasis to CAAs. We have evaluated the precisions with which these modalities diagnose the CAAs pertaining to origin and course of the related artery.
METHODS
For this registry, a retrospective review of patients, who underwent coronary imaging at a single-center over consecutive 2 years was performed. Patients diagnosed to have any CAA either on ICA or MDCTA were retrospectively included in the registry. Their status of evaluation and results on other imaging modalities, i.e. MDCTA or ICA respectively was checked if those were performed. Then the results of detection of CAA and their course delineations were compared.
ICA was performed in flatpanel cath-labs (Philips Medical systems, Nederland B.V.) under mono-plane fluoroscopy from femoral or radial arterial route. Anomalous separate origins of left anterior descending (LAD) and Circumflex arteries from left-sinus was revealed on left coronary angiogram in left anterior-oblique view with caudal angulation. The anomalous course of ACAOS was determined on the basis of ‘dot’ and ‘eye’ signs in the right anterior-oblique view. Myocardialbridge was defined as systolic constriction of the vessel of interest. Coronary-fistulae were detected from visualization of communication of coronary artery with any chamber.
MDCTA
Coronary MDCTA was performed with 128 slice CT scan (Somatom definition AS+ CT scanner machine, Siemens Healthcare, USA) in the supine position. Initially, a native, retrospectively Electrocardiogram (ECG)-triggered scan for coronary artery calcium-scoring was performed. It was followed by a contrast-enhanced, retrospectively ECG-gated coronary MDCTA. We used bolus-tracking technique to calculate the peak of contrast enhancement time. A bolus of contrast (350 mg/ml, Omnipaque; GE Healthcare) was injected intravenously at a dose of 1.5 ml/kg with dual-head power injector. It was followed by 10-20ml of saline-flush. Axial-images were obtained with 0.6mm slice thickness and 0.3mm recon increments with a medium sharp convolution kernel (B26). The retrospective ECG-gating was used for reconstruction. Out of initially obtained raw-data sets, standardized image reconstructions were performed at 5% increments of the R-R interval using a single-segment algorithm. Additional reconstructions throughout the whole cardiaccycle were also performed, if necessary. All acquired MDCTA images were transferred to a dedicated CT 3-dimensional post-processing work-station (Leonardo, Siemens Healthcare, Malvern, USA). Axial and curved multi-planar reformatted images, Maximum Intensity Projections, and Volume Rendered images were analyzed for the determination of the origin and course of coronaries, the take-off angles from the aorta, and size of the orifice. (Figures 1, 2)
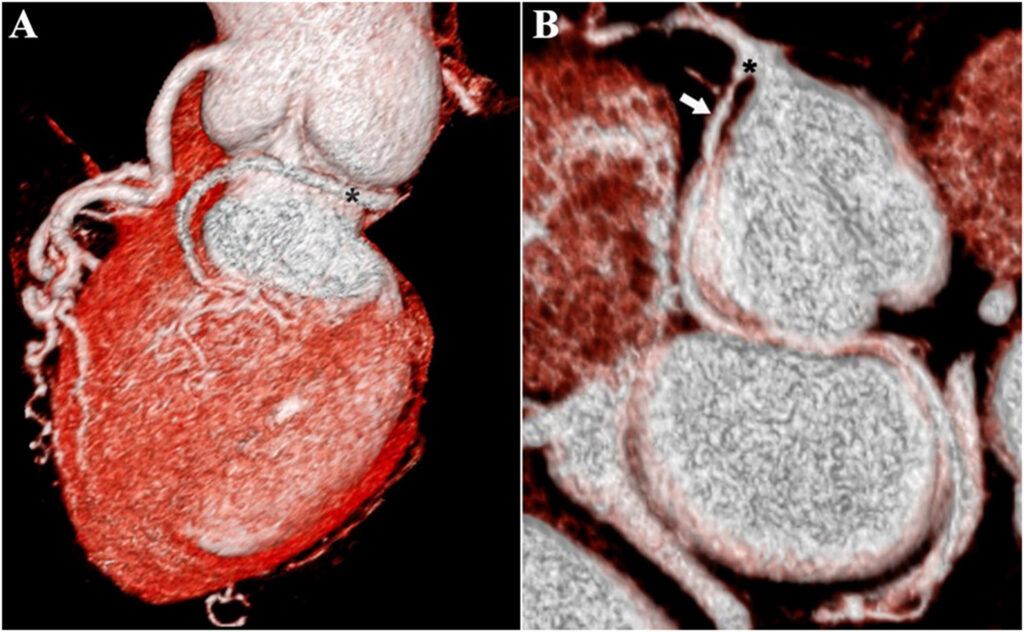
A: Volume rendered 3-dimensional image showing anomalous Circumflex artery (*)
B: Colored Maximum-Intensity Projections showing anomalous Circumflex artery (↘) with a branch-like origin (*)

A: Volume-rendered 3-dimensional image showing anomalous Right coronary artery (*)
B: Maximum-intensity projections showing Right coronary artery with a narrow take-off angle (↗)
C: Volume-rendered 3-dimensional image showing Right coronary artery (*) with a separate origin and a narrow take-off angle (↑)
Anomalies were defined and classified depending on anomalous origin and vessel course and the dependent myocardial territory, according to Angelini.4 Diagnosis of CAA was considered as proper detection of any particular CAA with the imaging modality of interest and was noted with every case. After origin, relation with surrounding anatomical structures between which the artery sequentially passes is termed as a course and its delineation was noted with every case. Scoring of anomalies was performed according to Rigatelli et al.5
Statistical analysis
Continuous data were expressed as mean ± standard-deviation, and were compared using the student paired- t-test (2-tailed). Categorical variables were expressed as percentages, and were compared (p-values) using the Chi-square test with Yatescorrection or Fisher’s exact-test. Correlations (r-values) between CAA-score- radiation-exposure, CAA score-contrast expenditure etc. were explored with Pearson’s correlation analysis. Sub-group analysis of those patients who underwent ICA and MDCTA both, was performed. Results on MDCTA were compared with results on ICA for analysis of sensitivity and specificity.
RESULTS
Prevalence
Indications for coronary evaluation commonly included angina, dyspnea or syncope in our patients. 257 patients (194 male, 63 female) out of 17,245 (12,608 male, 4637 female) coronary artery evaluations were found to have CAAs, at a prevalence of 1.49%. The prevalence of CAAs in males (1.538%) and in females (1.359%) equivalent (p=0.43). Out of these 257 patients, 40 were pediatric patients including 15 infants. Fifty-two patients were young adults (12-40-years) and 165 were more than 40 years. Association with other congenital anomalies was there in 28 patients. Out of 17,245 patients; 16,828 underwent ICA and 178 patients had their CAA primarily diagnosed on ICA. MDCTA was performed in 374 patients and 38 patients had their CAA primarily diagnosed on it. ICA and MDCTA both were performed in 186 patients.
Anatomical Classification
Amongst the patients with CAA, 88.72% had anomalies of origin and/or course; while, 11.3% had anomalies of termination or anastomosis.
Separate origins of LAD and Circumflex arteries from Left-sinus
Separate origin of LAD and Circumflex arteries from left-sinus was the commonest CAA (0.319%) in entire population and in elderly population (>40years) (29.7% of anomalies in that age-group). Amongst 55 patients with this anomaly, detected on ICA and/or MDCTA; ICA was performed in all 55. It detected the anomaly in 54 patients and in the remaining one it was already identified in MDCTA. Origin of LAD and malignant intramural course were not identified in one patient with ICA, but were delineated on MDCTA. Amongst those 55 patients, 38 patients underwent MDCTA. Sub-group analysis of 38 patients, who underwent MDCTA and ICA both, showed that ICA has sensitivity of 94.7% and specificity of 100%. MDCTA has sensitivity and specificity of 100%. For diagnosis of anomalous separate origins of LAD and Circumflex arteries; ICA and MDCTA had no significant difference (p=1.0).
Anomalous location of coronary ostium within or near proper aortic sinus
The commonest anomaly in young adults (12-40years) was anomalous high origin of coronary artery near proper sinus. Thirty-four patients had this anomaly, detected on ICA and/or MDCTA. ICA was performed in 20 patients amongst those. It detected the anomaly in only 11 patients. MDCTA was performed in 31 out of 34 patients. It diagnosed the anomaly in all those patients. Sub-group analysis showed that, 17 patients underwent ICA and MDCTA both. ICA has 52.9% and MDCTA has 100% sensitivity. Both, modalities have specificity of 100%. For detection of anomalous origin of from similar sinus, MDCTA was better than ICA (p=0.0001).
Anomalous location of coronary ostium outside normal coronary aortic sinuses
Twenty-three patients had such an anomaly where an anomalous coronary artery was arising from non-coronary cusp, ascending aorta, pulmonary artery or elsewhere. ICA was performed in 17 patients and MDCTA was performed in 14 patients. Eleven patients underwent ICA and MDCTA both on sub-group analysis. ICA has 81.8% and MDCTA has 100% sensitivity. Both modalities have 100% specificity.Anomalous Coronary Artery from the Opposite Sinus
It is group of CAAs comprising of many entities. Second mostcommon CAA was anomalous origin of Circumflex artery from the right-sinus (0.231%) in the entire study-population. The commonest anomaly in the entire pediatric-group (<12years) was ACAOS (42% of CAAs of that age-group). The 2nd most-common anomaly in elderly population (>40years) was anomalous origin of Circumflex artery from Right-sinus (17.58% of CAA of that age-group). Amongst 88 patients with ACAOS detected on ICA and/or MDCTA, ICA was performed in 79 patients. It detected the anomaly in 76 patients. However, it delineated the course properly in 73 patients only. On the contrary, MDCTA detected and delineated the course in all 74 patients in whom it was performed. In one patient, a non-specific view of ICA reported a high origin of Right coronary artery from ascending aorta. However, MDCTA has precisely shown that, it was arising from ascending aorta just above left-sinus. On subgroup analysis, 73 patients underwent both ICA and MDCTA. ICA had sensitivity of 95.9% for diagnosis, 91.8% for course delineation and specificity of 100% for both. MDCTA has 100% sensitivity and specificity. For ACAOS, the difference between diagnostic accuracy of ICA and MDCTA was not significant (p=0.49). Proper course delineation was significantly better with MDCTA than with ICA (p=0.05).Anomalies of termination
The third most-common anomaly was coronary-fistula (0.203%). It was the commonest anomaly in pediatric-group (1-12 years) (52% of CAAs of that age-group) and second most-common CAA in entire pediatric age-group (0-12 years) (37.5% of CAAs of that age-group). Thirty-five patients had coronary-fistulae on ICA or MDCTA. Thirty-four out of those underwent ICA. Fistula was diagnosed in all those with ICA but the proper course was delineated in only 31. MDCTA was performed in 33 patients. The CAA was diagnosed in 32 amongst those. The course of fistulous tract was delineated in all those. Thirty-one patients underwent ICA and MDCTA both on subgroup analysis. ICA had sensitivity and specificity of 100%. MDCTA had sensitivity of 96.8% and specificity 100%. ICA and MDCTA had no difference, for diagnosis and course delineation of termination anomalies, (p=0.49 and 0.61 respectively). However, course delineation was numerically better with MDCTA than with ICA.
Anomalous anastomotic vessels
For diagnosis of 3 such patients, ICA and MDCTA both were performed in all. ICA had higher sensitivity (100%) and equal specificity (100%) compared to MDCTA (66.67% and 100% respectively). For detection and course delineation, the difference between MDCTA and ICA was statistically insignificant (p=1.0).Comparisons
Mean heart-rate during MDCTA (74.37 ± 8.21/minute) was significantly lower than that during ICA, (85.44 ± 15.23/minute) (p=0.0001). Per-procedural need of beta-blockers to control heart-rate was significantly higher with MDCTA than that with ICA (p< 0.0001). Radiation-exposure with ICA (7.3 ± 2mSv) was lower than that with MDCTA (14.5 ± 3mSv) (p< 0.0001). Radiation-exposure for ACAOS was more than that for other anomalies with ICA (p< 0.001), but not with MDCTA (p=0.18). In case of ICA, radiation-exposure correlated with CAA-score (r=0.3). Furthermore, radiation-exposure correlated with CAA score for anomalies of origin and course even more intensely (r=0.6). In case of MDCTA, radiation did not correlate with CAA-score (r=-0.019). Mean contrast-expenditure during ICA (65.55±19.9 ml) and MDCTA (63.15±15.6 ml) were not significantly different (p=0.52). Contrast-expenditure during ICA in adults correlated with CAA-score (r=0.42). That during MDCTA did not correlate with CAA score (r=-0.04).DISCUSSION
Prevalence
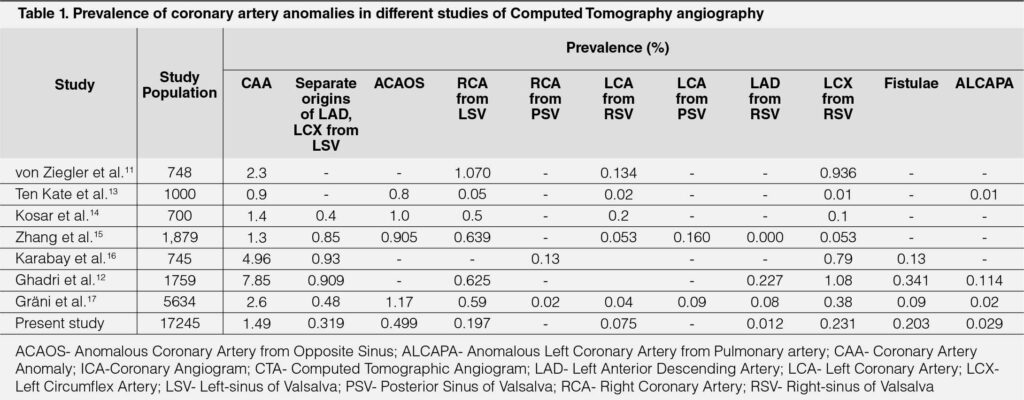
Historically, CAAs are seen in approximately 1% of the general population on ICA-based studies. Necropsy studies report even lower numbers. A study with precise criteria for CAAs reported a 5.64% prevalence,6 which was higher than the usual prevalence derived from ICA based reports, but comparable to one of the initial studies using 64-slice CT, reporting a prevalence of 7.9% of CAA of origin and course6 and dual-source CT-based study reporting 7% prevalence of CAA including fistulae.7 We reported 1.49% prevalence of CAAs. This is quite similar to that observed in large angiographic series,6, 8 large MDCTA studies with 4-/16-slice CT in 1758 patients, 64-slice CT in 1495 patients and coronary CTA studies reporting prevalence of 0.6-1.3%.2, 8 Even such large studies may not reflect general population as only symptomatic patients with indications for coronary angiography were considered. Cademartiri et al. reported a 1.5% prevalence of ACAOS, on MDCTA in 543 patients.9 A recent dual-source CTA based study, including patients referred for CAA, detected on ICA and preoperative patients of congenital heart-disease reported the prevalence of 3.7%.10 Ziegler et al reported that of 3.2%.11 Ghadri et al. showed that the prevalence of CAAs is substantially higher with CTA than that with ICA, suggesting a possible underestimation of CAAs on ICA.12 (Table 1)4, 8, 11–17
Anomalies of origin and course
In our study, MDCTA was better than ICA for the diagnosis of anomalous location of coronary ostium within or near proper aortic sinus. Both were equivalent for identification of separate origins of LAD and Circumflex arteries from left-sinus, ACAOS and myocardial-bridge. However, MDCTA was better than ICA for proper course delineation. For anomalies of coronary structure like myocardial-bridge; depth and length, which are important for risk-stratification,5 are better delineated with MDCTA than ICA.
Anomalies of termination and anastomosis
Due to the complex 3-dimensional natures of these anomalies; ICA often incompletely defines the anatomical course. ICA for fistulous anomalies may require a catheter in the right ventricular outflow. In addition, views with multiple angulations may be required for proper course definition. Reliable, complete, noninvasive assessment or indeed exclusion of CAAs is therefore desirable. There was no significant difference between ICA and MDCTA for identification of a CAAs of termination and anastomosis in our study.
Invasive Coronary Angiography
It is conventionally the imaging test of choice and importance for the diagnosis, characterization and classification of CAAs. Because of its invasiveness, relatively low spatial resolution, absence of soft tissue information, the inability to define the potentially complex three-dimensional anatomical course of these anomalies in relation to surrounding structures due to the limited number of two-dimensional projections limiting the accurate diagnosis of CAA by ICA; it has been progressively replaced by CTA.10, 18 Misinterpretation of course is reported in up to 50% of cases with ICA.2 In a study, ICA alone achieved correct identification of the abnormality in only 53% (p=0.016).1 The presence of a CAA origin is sometimes only suspected on ICA, particularly in cases of unsuccessful engagement or visualization of an artery. Additionally, the declining use of pulmonary catheters during ICA makes it difficult to discern the anterior versus posterior trajectory of the artery of interest. The information from ICA pertains to the coronary lumen alone. ICA has well-known limitations in characterizing ACAOS, as large registry data have shown that the initial course of ACAOS vessels may not be accurately identified by ICA in up to 40% of cases.19
Multi-detector Computed Tomography angiography
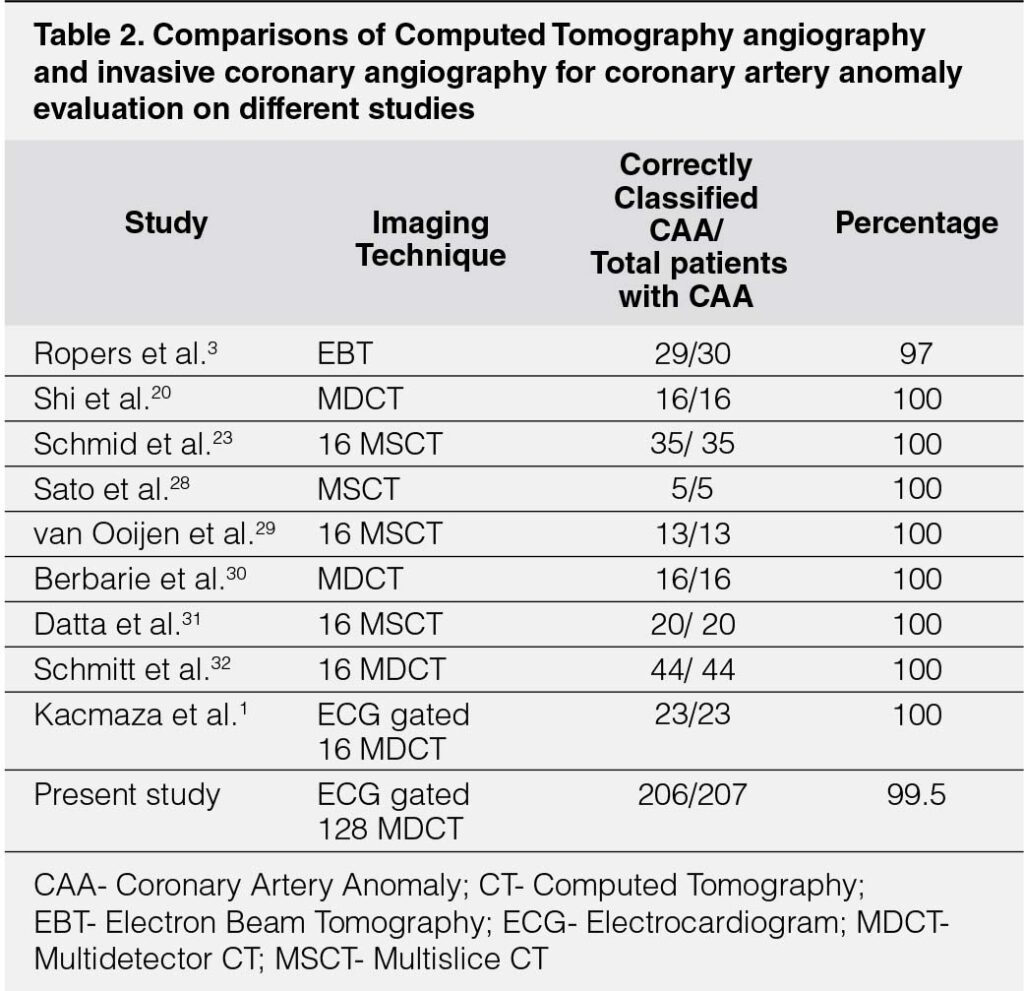
Early reports of using CTA to evaluate CAAs have emphasized Electron-beam CT. MDCTA offers numerous advantages making it the gold-standard to study CAAs.12, 18, 20 It offers the best performance in terms of excellent spatial resolution demonstrating precise origin and course in 3-dimensional relation to the surrounding cardiac and non-cardiac structures, acquisition time and image contrast, benefitting characterization of CAAs compared to ICA.1, 3, 21-23 It provides anatomic clues for high-risk CAAs and is more widely applicable for population studies.18, 21, 24 However, its widespread use is limited by the doses of radiation and contrast, particularly taking into account that most patients are young.22 CT scanners with 128 and 256-slice detectors can achieve an improved temporal resolution of up to 83ms10, 21 and 75ms respectively. It is now possible to have high-quality imaging without β-blockers, even in patients with high heart-rates, particularly with modern protocols, allowing MDCTA with a radiation-dose substantially lower than that of ICA.25, 26 Therefore, now, MDCTA is a recommended first-line imaging modality to assess the suspected or even diagnosed CAAs.20 The introduction of ECG-gating and post-processing rendering systems has resulted in significantly improved image-quality, reduced acquisition-time, and reduced radiation-exposure.25, 27 In comparison with ICA; Electron-beam CT and MDCTA correctly identify all normal controls and all patients with CAAs. In a study of 30 patients including ACAOS and coronary cameral fistulae, the anatomic course of the CAA was correctly classified with 97% accuracy.3 It demonstrated CT as a reliable non-invasive technique to define CAAs and their course.3 (Table 2)1, 3, 20, 23, 28–32
MDCTA is a part of hybrid imaging combining CTA and nuclear Imaging- Single-Photon Emission CT as an option to cardiac Magnetic Resonance for matching between myocardial territories supplied by the CAA and ischemia to determine causative role of CAA in ischemia.19, 33, 34 It appears mandatory before surgery is indicated.18
Prognosis and adverse cardiac events in patient with CAA with interarterial course are associated with several MDCTA findings. In one study, apart from stenosis, interarterial length more than 10mm on CTA was a predictor of event.24 Surgical intervention may be required for ACAOS with the malignant intramural course. The choice of surgical technique depends mostly on the origin and the extent of the intramural tract.18, 35 In a recent study, 44% of patients with CAA diagnosed on prior ICA were referred for CTA to achieve more refined characterization of ACAOS.22 Minimum and maximum diameters of coronary artery, shape of ostium (slit-like versus normal or oval), proximal vessel narrowing length >5.4mm suggesting intramural course, acute take-off angle <45° on multi-planar axial reconstruction at the level of ostium, level of vessel take-off (at, above or below the aortic-commissure) and ostium type (separate versus shared, or branch-vessel) on CTA predicted ischemia, need of surgical revascularization in patients with ACAOS and are of importance in surgical planning as per Congenital Heart Surgeons Society registry.21 In the presence of a short or absent intramural course, the options are either direct re-implantation of the anomalous vessel in the correct aortic sinus or osteoplasty. In cases with long intramural-course, the technique of choice is unroofing.18, 35, 36 Aortic insufficiency due to inadvertent incision of the aortic-commissure or damage to the coronary wall are the risks,18 which are avoidable by proper pre-operative knowledge of course. In the case of inter-arterial course, pulmonary translocation is an alternative.18, 35, 36 With additional significant atherosclerotic stenosis of target vessel or with absence of other available options, coronary artery bypass-grafting is an option.18, 35, 36 So, all these imply that origin, length and extent of intramural and inter-arterial course, relation to pulmonary-artery and aortic-valve are important for surgical planning to avoid complications. Proper delineation of course after anomalous origin was significantly better with MDCTA than with ICA with our study. So, for surgical planning MDCTA is more appropriate than ICA with ACAOS.
The choice of technique for fistula-closure like surgical-ligation at the drainage site for more complex fistulae or percutaneous closure with coils, plugs or umbrella-devices in cases of a single non-tortuous fistulae depends on the morphology, course, tortuosity and an aneurysmal dilatation of the afferent vessel which are delineated with MDCTA and ICA equally in our study with numerically better delineation of surrounding structures with MDCTA.
CONCLUSION
Though ICA is considered a gold-standard for evaluation of CAA, MDCTA is a good alternative for diagnosis. In most anomalies, 3-dimensional course delineation is better with MDCTA as compared to ICA. It is helpful in planning of management of most of CAAs. Radiation and contrast exposure with MDCTA do not correlate with complexity of anomaly in contrast to ICA.
KEY WORDS
Coronary artery anomalies; Invasive Coronary Angiography; Multidetector row-Computed Tomography angiography; Radiation Exposure
REFERENCES
1. Kacmaza F, Ozbulbulb NI, Alyana O, Madena O, Demira AD, Balbaya Y, Erbaya AR , Ataka R, Senena K, Olcerb T, Ilkayc E. Imaging of coronary artery anomalies: the role of multidetector computed tomography. Coron Artery Dis 19:203-9, 2008. Doi:10.1097/MCA.0b013e3282f528f1
CrossRef Pubmed
2. Kardos A, Babai L, Rudas L, Gaál T, Horváth T, Tálosi L, Tóth K, Sárváry L, Szász K. Epidemiology of congenital coronary artery anomalies: a coronary arteriography study on a central European population. Cathet Cardiovasc Diag 42:270-5, 1997. Doi:10.1002/(sici)1097-0304(199711)42:3<270::aid-ccd8>3.0.co;2-9 CrossRef Pubmed
3. Ropers D, Moshage W, Daniel WG, Jessl J, Gottwik M, Achenbach S. Visualization of coronary artery anomalies and their anatomic course by contrast-enhanced electron beam tomography and three-dimensional reconstruction. Am J Cardiol 87:193-7, 2001.Doi:10.1016/s0002-9149(00)01315-1 CrossRef Pubmed
4. Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation 115:1296-305, 2007. Doi:10.1161/CIRCULATIONAHA.106.618082 CrossRef Pubmed
5. Rigatelli G, Rigatelli A, Cominato S, Panin S, Nghia NT, Faggian G. A clinical-angiographic risk scoring system for coronary artery anomalies. Asian Cardiovasc Thorac Ann 20:299-303, 2012. Doi:10.1177/0218492312437880 CrossRef Pubmed
6. Angelini P. Coronary artery anomalies—current clinical issues: definitions, classification, incidence, clinical relevance, and treatment guidelines. Tex Heart Inst J 29:271-8, 2002. PMID:12484611; PMCID: PMC140289
Pubmed
7. De Jonge GJ, Van Ooijen PMA, Piers LH, Dikkers R, Tio RA, Willems TP, van den Heuvel AFM, Zijlstra F, Oudkerk M. Visualization of anomalous coronary arteries on dual-source computed tomography. Eur Radiol 18:2425-32, 2008. Doi:10.1007/s00330-008-1110-y CrossRef Pubmed
8. Yamanaka O, Hobbs RE. Coronary artery anomalies in 126,595 patients undergoing coronary arteriography. Cathet Cardiovasc Diagn 21:28-40, 1990. Doi:10.1002/ccd.1810210110 CrossRef Pubmed
9. Cademartiri F, La Grutta L, Malagò R, Alberghina F, Meijboom WB, Pugliese F, Maffei E, Palumbo AA, Aldrovandi A, Fusaro M, Brambillia V, Coruzzi P, Midiri M, Mollet NRA, Krestin GP. Prevalence of anatomical variants and coronary anomalies in 543 consecutive patients studied with 64-slice CT coronary angiography. Eur Radiol 18:781-91, 2008. Doi:10.1007/s00330-007-0821-9 CrossRef Pubmed
10. Chaosuwannakit N. Anatomical variants and coronary anomalies detected by dual-source coronary computed tomography angiography in North-eastern Thailand. Pol J Radiol 83:e372-8, 2018. Doi:10.5114/pjr.2018.78420 CrossRef Pubmed
11. Von Ziegler F, Pilla M, McMullan L, Panse P, Leber AW, Wilke N, Becker A. Visualization of anomalous origin and course of coronary arteries in 748 consecutive symptomatic patients by 64-slice computed tomography angiography. BMC Cardiovasc Disord 9:54, 2009. Doi:10.1186/1471-2261-9-54 CrossRef Pubmed
12. Ghadri JR, Kazakauskaite E, Braunschweig S, Burger IA, Frank M, Fiechter M, Gebhard C, Fuchs TA, Templin C, Gaemperli O, Luscher TF, Schmied C, Kaufmann PA. Congenital coronary anomalies detected by coronary computed tomography compared to invasive coronary angiography. BMC Cardiovasc Disord 14:81, 2014. Doi:10.1186/1471-2261-14-81 CrossRef Pubmed
13. Ten Kate GJR, Weustink AC, de Feyter PJ. Coronary artery anomalies detected by MSCT-coronary angiography in the adult. Neth Heart J 16:369-75, 2008. Doi:10.1007/BF03086181 CrossRef Pubmed
14. Kosar P, Ergun E, Ozturk C, Kosar U. Anatomic variations and anomalies of the coronary arteries: 64-slice CT coronary angiographic appearance. Diagn Interv Radiol 15:275-83, 2009. Doi:10.4261/1305-3825.DIR.2550-09.1 Pubmed
15. Zhang LJ, Yang GF, Huang W, Zhou CS, Chen P, Lu GM. Incidence of anomalous origin of coronary artery in 1879 Chinese adults on dual-source CT angiography. Neth Heart J 18:466-70, 2010. Doi:10.1007/BF03091817 CrossRef Pubmed
16. Karabay KO, Yildiz A, Geceer G, Uysal E, Bagirtan B. The incidence of coronary anomalies on routine coronary computed tomography scans. Cardiovasc J Afr 24:351-4, 2013. Doi:10.5830/CVJA-2013-066 CrossRef Pubmed
17. Gräni C, Benz DC, Schmied C, Vontobel J, Possner M, Clerc OF, Mikulicic F, Stehli J, Fuchs TA, Pazhenkottil AP, Gaemperli O, Kaufmann PA, Buechel RR. Prevalence and characteristics of coronary artery anomalies detected by coronary computed tomography angiography in 5634 consecutive patients in a single centre in Switzerland. Swiss Med Wkly. April 28, 2016. Doi:10.4414/smw.2016.14294 CrossRef Pubmed
18. Gentile F, Castiglione V, De Caterina R. Coronary Artery Anomalies. Circulation 144:983-96, 2021. Doi:10.1161/CIRCULATIONAHA.121.055347 CrossRef Pubmed
19. Barriales-Villa R, Morís C, Sanmartín JC, Fernández E, Pajín F, Ruiz Nodar JM. Anomalous coronary arteries originating in the contralateral sinus of Valsalva: registry of thirteen Spanish hospitals (RACES). Rev Esp Cardiol 59:620-3, 2006. PMID: 16790205 Pubmed
20. Shi H, Aschoff AJ, Brambs HJ, Hoffmann MHK. Multislice CT imaging of anomalous coronary arteries. Eur Radiol 14:2172-81, 2004. Doi:10.1007/s00330-004-2490-2 CrossRef Pubmed
21. Cheezum MK, Ghoshhajra B, Bittencourt MS, Hulten EA, Bhatt A, Mousavi N, Shah NR, Valente AM, Rybicki FJ, Steigner M, Hainer J, MacGillivray T, Hoffmann U, Abbara S, Di Carli MF, DeFaria Yeh D, Landzberg M, Liberthson R, Blankstein R. Anomalous origin of the coronary artery arising from the opposite sinus: prevalence and outcomes in patients undergoing coronary CTA. Eur Heart J Cardiovasc Imaging 18:224-35, 2017. Doi:10.1093/ehjci/jev323 CrossRef Pubmed
22. Villa AD, Sammut E, Nair A, Rajani R, Bonamini R, Chiribiri A. Coronary artery anomalies overview: The normal and the abnormal. World J Radiol 8:537-55, 2016. Doi:10.4329/wjr.v8.i6.537 CrossRef Pubmed
23. Schmid M, Achenbach S, Ludwig J, Baum U, Anders K, Pohle K, Daniel WG, Ropers D. Visualization of coronary artery anomalies by contrast-enhanced multi-detector row spiral computed tomography. Int J Cardiol 111:430-5, 2006. Doi:10.1016/j.ijcard.2005.08.027 CrossRef Pubmed
24. Ashrafpoor G, Danchin N, Houyel L, Ramadan R, Belli E, Paul JF. Anatomical criteria of malignancy by computed tomography angiography in patients with anomalous coronary arteries with an interarterial course. Eur Radiol 25:760-6, 2015. Doi:10.1007/s00330-014-3454-9 CrossRef Pubmed
25. Herzog BA, Wyss CA, Husmann L, Gaemperli O, Valenta I, Treyer V, Landmesser U, Kaufmann PA. First head-to-head comparison of effective radiation dose from low-dose 64-slice CT with prospective ECG-triggering versus invasive coronary angiography. Heart 95:1656-61, 2009. Doi:10.1136/hrt.2008.162420 CrossRef Pubmed
26. Ghadri JR, Küest SM, Goetti R, Fiechter M, Pazhenkottil AP, Nkoulou RN, Kuhn FP, Pietsch C, von Schulthess P, Gaemperli O, Templin C, Kaufmann PA. Image quality and radiation dose comparison of prospectively triggered low-dose CCTA: 128-slice dual-source high-pitch spiral versus 64-slice single-source sequential acquisition. Int J Cardiovasc Imaging 28(5):1217-1225, 2012. Doi:10.1007/s10554-011-9921-3 CrossRef Pubmed
27. Fuchs TA, Stehli J, Bull S, Dougoud S, Clerc OF, Herzog BA, Buechel RR, Gaemperli O, Kaufmann PA. Coronary computed tomography angiography with model-based iterative reconstruction using a radiation exposure similar to chest X-ray examination. Eur Heart J 35(17):1131-1136, 2014. Doi:10.1093/eurheartj/ehu053 CrossRef Pubmed
28. Sato Y, Inoue F, Matsumoto N, Tani S, Takayama T, Yoda S, Kunimasa, T, Ishii N, Uchiyama T, Saito S, Tanaka H, Furuhashi S, Takahashi M, Koyama Y. Detection of anomalous origins of the coronary artery by means of multislice computed tomography. Circ J. 69:320-4, 2005. Doi:10.1253/circj.69.320 CrossRef Pubmed
29. Van Ooijen PMA, Dorgelo J, Zijlstra F, Oudkerk M. Detection, visualization and evaluation of anomalous coronary anatomy on 16-slice multidetector-row CT. Eur Radiol 14:2163-71, 2004. Doi:10.1007/s00330-004-2493-z CrossRef Pubmed
30. Berbarie RF, Dockery WD, Johnson KB, Rosenthal RL, Stoler RC, Schussler JM. Use of Multislice Computed Tomographic Coronary Angiography for the Diagnosis of Anomalous Coronary Arteries. Am J Cardiol 98:402-6, 2006. Doi:10.1016/j.amjcard.2006.02.046 CrossRef Pubmed
31. Datta J, White CS, Gilkeson RC, Meyer CA, Kansal S, Jani ML, Arildsen RC, Read K. Anomalous Coronary Arteries in Adults: Depiction at Multi–Detector Row CT Angiography. Radiology 235:812-8, 2005.Doi:10.1148/radiol.2353040314 CrossRef Pubmed
32. Schmitt R, Froehner S, Brunn J, Wagner M, Brunner H, Cherevatyy O, Gietzen F, Christopoulos G, Kerber S, Fellner F. Congenital anomalies of the coronary arteries: imaging with contrast-enhanced, multidetector computed tomography. Eur Radiol 15:1110-21, 2005. Doi:10.1007/s00330-005-2707-z CrossRef Pubmed
33. Gräni C, Benz DC, Possner M, Clerc OF, Mikulicic F, Vontobel J, Stehli J, Fuchs TA, Pazhenkottil AP, Gaemperli O, Kaufmann PA, Buechel RR. Fused cardiac hybrid imaging with coronary computed tomography angiography and positron emission tomography in patients with complex coronary artery anomalies. Congenit Heart Dis 12(1):49-57, 2017. Doi:10.1111/chd.12402 CrossRef Pubmed
34. Uebleis C, Groebner M, Von Ziegler F, Becker A, Rischpler C, Tegtmeyer R, Becker C, Lehner S, Haug AR, Cumming P, Bartenstein P, Franz WM, Hacker M. Combined anatomical and functional imaging using coronary CT angiography and myocardial perfusion SPECT in symptomatic adults with abnormal origin of a coronary artery. Int J Cardiovasc Imaging 28(7):1763-1774, 2012. Doi:10.1007/s10554-011-9995-y CrossRef Pubmed
35. Harky A, Noshirwani A, Karadakhy O, Ang J. Comprehensive literature review of anomalies of the coronary arteries. J Card Surg 34(11):1328-1343, 2019. Doi:10.1111/jocs.14228 CrossRef Pubmed
36. Brothers JA, Frommelt MA, Jaquiss RDB, Myerburg RJ, Fraser CD, Tweddell JS. Expert consensus guidelines: Anomalous aortic origin of a coronary artery. J Thorac Cardiovasc Surg 153(6):1440-1457, 2017. Doi:10.1016/j.jtcvs.2016.06.066 CrossRef Pubmed
Copyright Information
