Original Article
Aug 2022, 31:1
First online: 3 August 2022
Original Article
A Case Series on Hypertrophic Cardiomyopathy with Midventricular Obstruction and Apical Aneurysm
Philippe Ryan K. Chung MD,1 Emma Y. Gaspar-Trinidad M.D,1 Tamara Louise J. Razon-Cuenza M.D, 1 Lucky R. Cuenza M.D1
ABSTRACT
Midventricular obstruction (MVO) is a rare phenotypic subvariant of hypertrophic cardiomyopathy (HCM). It occurs in 1-10% of patients and due to its rarity, its clinical progression and natural history has not yet been extensively studied. However, the available data has shown it to have worse clinical outcomes and prognosis. While reports on hypertrophic cardiomyopathy has been published in the Philippines, there has been no data on this particular variant in the local literature.
We identified 3 cases of hypertrophic cardiomyopathy with midventricular obstruction. 2 cases were initially managed as a case of ischemic heart disease who were ruled out after undergoing cardiac magnetic resonance. One case was initially admitted due to a stroke however, an incidental finding on 2D Echo noted the presence of HCM. He also underwent cardiac magnetic resonance which revealed the full extent of his disease. All 3 patients were also found to have apical aneurysms due to ischemia from the pressure coming from the obstruction.
Hypertrophic cardiomyopathy is the most common genetic cardiovascular disease but its subvariants are rare and difficult to diagnose. Apical aneurysms are also often missed on 2D Echo. However, cardiac magnetic resonance provides increased detection of the disease with better detailing of the extent and severity. It could also provide information regarding prognostication with regards to arrhythmias and sudden cardiac death. More data however is needed so new avenues for research are open on its application.
INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is a common genetic cardiac disease, affecting 1 in 500 in the general population or a prevalence of 0.2%. An autosomal dominant mutation in the MYH7 gene encoding for the sarcomere protein β-myosin heavy chain and myosin protein binding C (MYBPC3) are the most common mutations. There are also sever l genotypic subtypes but all of them results to altered sarcomere function and contraction. In turn, this leads to compromised cardiomyocyte energetic balance, triggering hypertrophy and adverse remodeling. This also affects the coronary microvasculature which can lead to LV dysfunction and failure.1-2
HCM patients can present in a myriad of ways and can be confusing. Some can be completely asymptomatic. Others can manifest with chest pain, dyspnea, palpitations or even syncope. The standard 12-lead ECG could show left ventricular hypertrophy, ST or T-wave abnormalities or pathological Q-waves. Atrial fibrillation is also the most common arrhythmia in HCM. Transthoracic echocardiography is central to the diagnosis of HCM where the hypertrophic myocardial segments are visualized, the pressure gradients and the diastolic function can be measured using continuous wave Doppler. New imaging modalities like cardiac magnetic resonance (CMR) help establish diagnosis in patients with a poor acoustic window. Myocardial fibrosis can also be demonstrated via late gadolinium enhancement.3
The 2014 European Society of Cardiology (ESC) Guidelines on the Diagnosis and Management of HCM has not made a recommendation regarding surgical therapy. However, surgical myectomy has been attempted and may present opportunities for treatment in high-risk cases. In the meantime, medical therapy includes beta-blockers or non-dihydropyridine calcium channel blockers for reducing heart rate and improving diastolic filling. Risk stratification using the HCM Risk SCD score has been recommended to determine the chance for sudden cardiac death. ICD implantation is advised for those with >6% estimated risk.3, 5
CASE #1
A 67 year-old hypertensive, diabetic male consulted due to sharp, exertional chest pain radiating to the nape and left axilla. He is hypertensive, diabetic and was a heavy smoker years before. He had a coronary angiogram in 2016 showing myocardial bridging along the mid-segment of the left anterior descending artery mild disease in the left main coronary artery and the left circumflex. He was also previously diagnosed with HCM in 2017 via CMR.
Upon arriving at the emergency room, his vital signs were as follows: blood pressure 120/70 mmHg, heart rate 98 beats/min, respiratory rate 24 breaths/min and axillary temperature of 36.5°C. Physical examination findings were unremarkable. His initial ECG showed atrial fibrillation in rapid ventricular response, left axis deviation and T wave inversions at V3-V6, leads I and aVL and a repeat done the following morning showed atrial flutter at 2:1 atrioventricular conduction but still with the same T wave inversion pattern. Serial biomarkers were initially normal but was later positive. His echocardiogram done on the 2nd hospital day showed a thickened left ventricular wall thickness measuring 1.9 cm and obliteration of the mid-LV cavity.
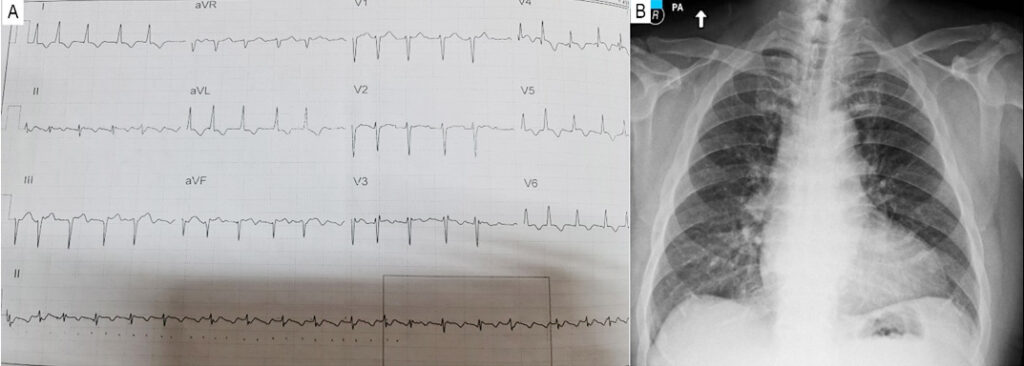
Figure 1
(A) 12-lead ECG taken at the ER demonstrating atrial fibrillation in rapid ventricular response, left axis deviation and T wave inversions at V3-V6, leads I and aVL (B) Chest X-ray showing an enlarged left ventricle.
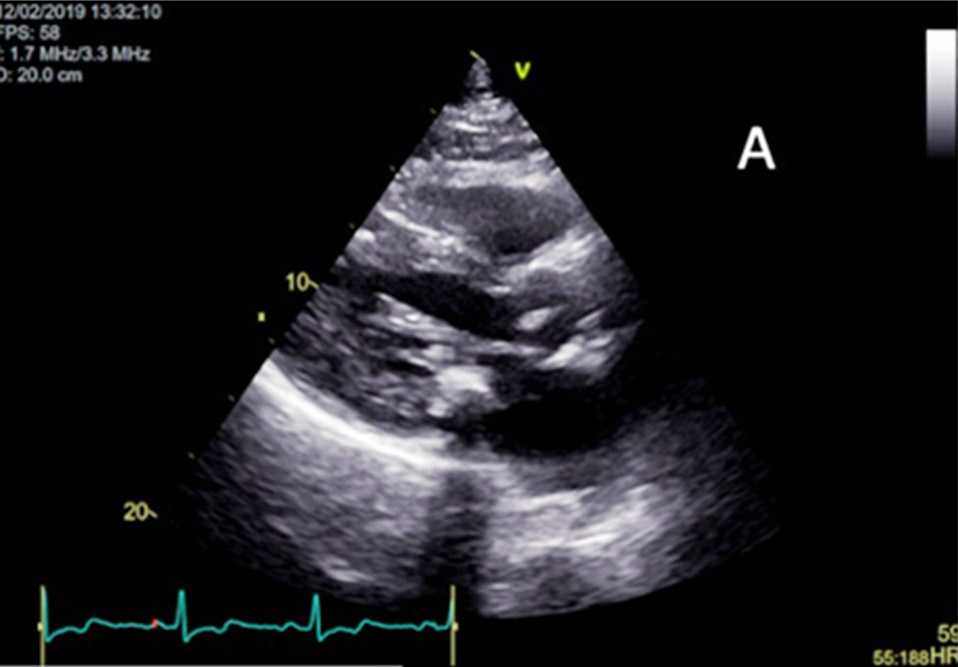
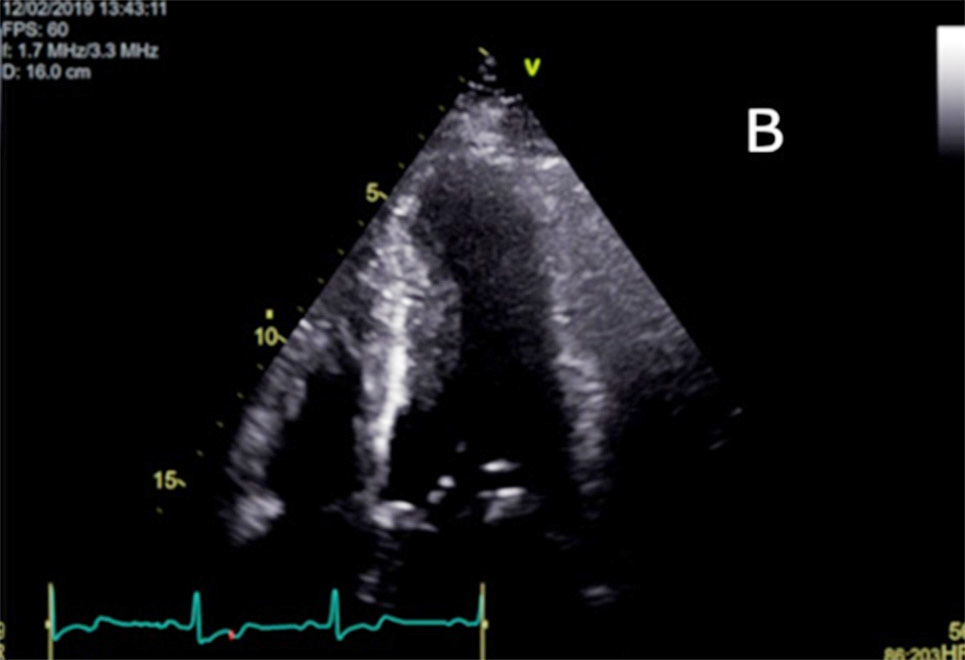

Figure 2
(A) Parasternal long axis image on 2D Echocardiogram showing hypertrophic mid ventricular segment.
(B) Apical 4-chamber image on 2D Echocardiogram also showing the hypertrophic midventricular segment and aneurysmal apical region.
(C) Doppler color flow showing a “lobster claw” flow profile described as an early high systolic velocities followed by an interruption of flow then a second systolic peak. The midventricular gradient is 57 mmHg.
Initial management focused on controlling his heart rate with intravenous Digoxin and oral Metoprolol tartrate. However, as serial cardiac troponins became elevated, the primary working impression shifted to Acute Coronary Syndrome. Later that day, electrical cardioversion was performed once transesophageal echocardiography ruled out the presence of intracardiac thrombi. Coronary angiography was considered but the patient’s chest pain appeared controlled and serial ECGs did not show progressive ST wave changes. The Heart Team opted to pursue a cardiac magnetic resonance (CMR) study instead which confirmed the presence of the midventricular hypertrophy measured at 2.6 cm as well as a thinned-out apical aneurysm. Furthermore, there was diffuse subendocardial to transmural late gadolinium enhancement in the mid and apical left ventricular segments after the obstruction. These findings were not present in his previous CMR. The coronary angiography was then called off with the elevated cardiac troponin levels attributed to the tachycardia from the atrial fibrillation and the hypertrophic cardiomyopathy. The patient was maintained on Metoprolol tartrate on top of his hypertension and diabetes medication. Risk stratification was done by an electrophysiologist, where he was found to be low-risk for sudden cardiac death based on the echocardiographic parameters but if high-risk if based on the CMR parameters. He was then discharged stable and on close follow-up.
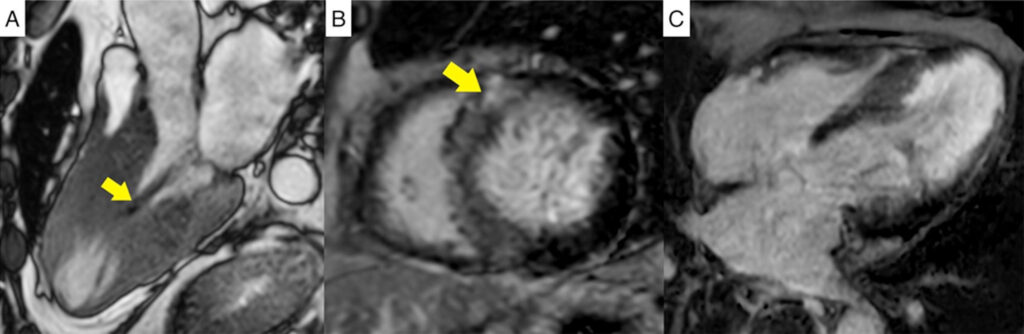
Figure 3
(A) 3-chamber steady-state free precession cine image in mid systole showing near obliteration of the mid ventricular cavity
due to asymmetric hypertrophy associated with a systolic stenotic jet (pointed by arrow) and apical aneurysm formation.
(B) Short axis phase-sensitive inversion recovery image at the mid ventricular level demonstrating non-ischemic late gadolinium enhancement in the hypertrophic septal segments and at the RV insertion points typical of HCM (pointed by arrow).
(C) 4-chamber phase-sensitive
inversion recovery image revealing ischemic pattern late gadolinium enhancement in the mid to apical segments.
CASE #2
A 64 year-old male came to the ER due to right sided weakness that first started a week prior and had been slowly progressing since along with difficulty speaking and dizziness. He denied any chest pains, dyspnea or palpitations. He is a known hypertensive for the past 15 years and only maintained on Telmisartan + HCTZ. He is also a chronic smoker and has a strong history for stroke in the family. Upon arriving at the ER, his BP was 200/100 mmHg, HR 90 bpm, RR 20 bpm, T 36.6C. His physical and neurologic exams were normal. A Cranial MRI/MRA revealed a small acute ischemic infarct of the left ventral pons hence he was on Aspirin, Atorvastatin and Citicholine. His blood pressure was controlled with the help of a Nicardipine drip. A 12L-ECG showed sinus rhythm with LVH. He was managed as a case of Acute CVD Infarct and was monitored in the ICU for the next several days without incident. A 2D Echo however, noted increased left ventricular relative wall thickness measuring at 1.8 cm with mid- LV cavity obstruction during systole. Once he was more stable and transferred out to the floors, a cardiac MRI was performed which revealed asymmetric hypertrophy most pronounced at the mid-septum with maximal wall thickness at 2.39 cm. There was a prominent distal apical cavity with akinesia of the true apical segment which became intense on late gadolinium enhancement at the subendocardial regions along with the mid-apical anterior, inferior and lateral wall segments. His risk of sudden cardiac death was low even when using the CMR measurements hence he was not referred for ICD implantation. Carvedilol was included in his discharge medications for stroke and hypertension.

Figure 4
(A) 12-lead ECG taken at the ER demonstrating sinus rhythm with left ventricular hypertrophy (B) Chest X-ray showing an enlarged left ventricle.

Figure 5
(A) Parasternal long axis view on 2D Echo showing the thickened mid ventricular segments.
(B) 4-chamber view on 2D Echo showing the thickened mid ventricular segment and the aneurysmal apical segments.
(C) Doppler color flow showing also demonstrating the “lobster claw” flow profile. The midventricular gradient is 8 mmHg.

Figure 6
(A) 3-chamber steady-state free precession image in mid systole showing near obliteration of the mid ventricular cavity due to asymmetric hypertrophy and apical aneurysm formation.
(B) Short axis phase-sensitive inversion recovery image at the mid ventricular level demonstrating non-ischemic late gadolinium enhancement in the hypertrophic septal segments typical of HCM (pointed by arrows).
(C) 4-chamber phase-sensitive inversion recovery image revealing ischemic pattern late gadolinium enhancement in the mid to apical segments.
CASE #3
A 41 year-old hypertensive male who was initially seen and worked up in a clinic outside of our institution. He is asymptomatic but had left atrial enlargement, left ventricular hypertrophy and intraventricular conduction delay on ECG. While mild septal hypertrophy measuring 1.7 cm was noted on 2D Echo, he was initially diagnosed and managed as Coronary Artery Disease instead of HCM. His treadmill stress echoes done in 2017 and 2018 were normal. There was no noted mid-cavity or outflow tract obstruction. A Tl-201 MPI done in 2019 showed severe inducible ischemia at the apex and apical inferior and lateral segments but a coronary angiogram showed angiographically normal coronary arteries. He sought second opinion with another cardiologist the following year and underwent cardiac magnetic resonance in our institution which revealed asymmetric septal hypertrophy involving the mid and apical segments with a maximal thickness 2.48 cm and nonischemic late gadolinium enhancement indicative of fibrosis. There was also noted apical dilatation, thinning and akinesia with superimposed subendocardial to transmural late gadolinium enhancement in the mid-apical segments after the mid-ventricular obstruction. He was low risk for sudden cardiac death and was prescribed Carvedilol for maintenance medications but was lost to follow-up after he went overseas for work.

Figure 7
A dual tracer SPECT using Tl-201 at rest then Tc99m injected at peak exercise demonstrating inducible ischemia at peak stress involving the apex and apical inferior and lateral segments. He underwent treadmill stress test using modified Bruce protocol and was able to accomplish 12.4 METS before stopping due to leg fatigue.
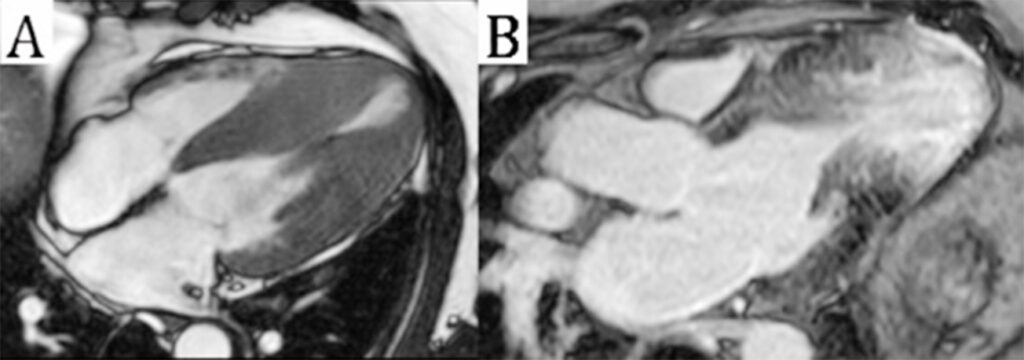
Figure 8
(A) 3-chamber steady-state free precession image in mid systole showing near obliteration of the mid ventricular cavity due to asymmetric hypertrophy associated with a systolic stenotic jet and apical aneurysm formation.
(B) 3-chamber phase-sensitive inversion recovery image revealing ischemic pattern late gadolinium enhancement in the mid to apical segments.
DISCUSSION
While HCM has become fairly common, information regarding midventricular obstruction (MVO) is scarce in the literature. Most of which are limited to individual case reports with the exception of the single center study done by Efthimiadis et al. where they took 423 HCM patients for a period of 7 years and found 34 had MVO. Endpoints such as death from any cause, CV death and sudden cardiac death were found to be higher in the MVO group. 11.8% of the MVO patients progressed to endstage heart failure and 26.5% of MVO patients developed left ventricular apical aneurysms while no one in the left ventricular outflow tract obstruction group developed them. At the same time, 97% develop symptoms compared to 85% and 67% respectively. The ischemia from the “burned-out” apex in particular, could have been the source of the patient’s chest pain. At the same time, the presence of the apical aneurysm is a result of the same pathophysiology as discussed above. Though there were no significant differences between those MVO patients with apical aneurysm and those without, it is reasonable to assume that the intracavitary gradients were higher in those who developed apical aneurysms.3
The role of CMR in different cardiac diseases has been evolving in recent years. When echocardiography becomes difficult, CMR provides good visualization of the cardiac anatomy including the dimensions of the left ventricle as well as functional parameters. In early cases of HCM, CMR offers greater spatial resolution and can identify mild septal hypertrophy with normal wall thickness on echo.4 Furthermore, its ability in evaluating myocardial tissue has proven invaluable in diagnosing HCM and in assessing for fibrosis and scarring. The interventricular septum is usually involved followed by the left ventricular free wall. Foci of late gadolinium enhancement are non-coronary in distribution and tend to occur in and around the hypertrophied segments. CMR has also been able to demonstrate the development of scarred, thin-walled left ventricular apical aneurysms. The ischemic pattern of injury at the aneurysmal segments can also be seen with late gadolinium enhancement and in the context of HCM, is related with adverse outcomes and events. The extent of fibrosis via gadolinium enhancement has already been associated with increased risk of SCD as such CMR has been advocated as a risk stratification tool in enabling decisions regarding ICD insertion. Use of late gadolinium enhancement in HCM patients correlated with increased risk of cardiac death, death from heart failure and all-cause mortality.8-9 Additionally, the presence of an apical aneurysm may be a marker for SCD risk as such ICD insertion had been recommended. However more evidence regarding this needs to be conducted. Furthermore, the presence of an apical aneurysm raises questions about the use of systemic anticoagulation. All these open up avenues for research with CMR and its prognostic significance.7-9
While CMR has been recommended in the 2014 ESC Guidelines, the recommendation says it should only come as a backup in case echocardiography cannot provide good visualization of the cardiac anatomy.3 For our patients, CMR has been invaluable in providing information that was missed out in echocardiography. The accuracy is evident in the difference in the measured dimensions. The difference in the dimensions were so stark that the risk stratification would change if the CMR values were used in the HCM Risk SCD score instead of the echo. The tissue was also adequately characterized with the late gadolinium enhancement of the non-ischemic hypertrophied segments and ischemic “burned-out” apex. The apical aneurysm was also well-visualized but was missed on the echo. Furthermore, a meta-analysis done by Wang et al. found that the HCM Risk SCD calculator had high specificity and negative predictive value which avoids overtreatment with ICD implantation however, had poor sensitivity and positive predictive value which leads to missing out high risk patients with HCM.10
All these result in poorer prognostication of the SCD calculator in predicting events. A recent editorial by Pellicia et al. highlighted the need for novel markers to supplement traditional factors leading to SCD. These include disorganized architecture and myocardial fibrosis identified using late gadolinium enhancement as well as apical aneurysms which are found more commonly on CMR.11 As more data and reports emerge, there could be an expansion of the role of CMR and its applications with respect to HCM. Pellicia et al. add that the guidelines of the ACC/AHA are being revised and updated in light of recent evidence.5, 11-12
CONCLUSION
Hypertrophic cardiomyopathy with a midventricular morphologic subtype is an uncommon variant of HCM that may lead to abnormalities and can present with chest pain and elevated Troponin, mimicking the presentation of other cardiac disorders. The diagnosis was confirmed though using the right imaging modalities and good clinical judgment. CMR has played an important role in these cases and more avenues in its application can be a subject for further research.
Keywords Hypertrophic Cardiomyopathy, Midventricular Obstruction, Cardiac Magnetic Resonance
REFERENCES
1. Olivotto I, Cecchi F, Poggesi C, Yacoub MH. Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ Heart Fail. 2012 Jul 1;5(4):535-46. doi: 10.1161/CIRCHEARTFAILURE.112.967026. PMID: 22811549. CrossRef Pubmed
2. Marian AJ, Braunwald E. Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res. 2017;121(7):749-770. doi:10.1161/CIRCRESAHA.117.311059 CrossRef Pubmed
3. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force
for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014 Oct 14;35(39):2733-79. doi: 10.1093/eurheartj/ehu284. Epub 2014 Aug 29. PMID: 25173338. CrossRef Pubmed
4. Valente AM, Lakdawala NK, Powell AJ, Evans SP, Cirino AL, Orav EJ, MacRae CA, Colan SD, Ho CY. Comparison of echocardiographic and cardiac magnetic resonance imaging in hypertrophic cardiomyopathy sarcomere mutation carriers without left ventricular hypertrophy. Circ Cardiovasc Genet. 2013 Jun;6(3):230-7. doi: 10.1161/CIRCGENETICS.113.000037. Epub 2013 May 20. PMID: 23690394; PMCID: PMC3974911. CrossRef Pubmed
5. Mershina, Elena & Blagova, Olga & Sinitsyn, Valentin & Pershina, Ekaterina. (2016). A case of hypertrophic cardiomyopathy with “burned-out” apex of the left ventricle due to mid-ventricular obstruction. Clinical Case Reports and Reviews. 2. 10.15761/CCRR.1000S2002. CrossRef
6. Kunkala MR, Schaff HV, Nishimura RA, Abel MD, Sorajja P, Dearani JA, Ommen SR. Transapical approach to myectomy for midventricular obstruction in hypertrophic cardiomyopathy. Ann Thorac Surg. 2013 Aug;96(2):564-70. doi: 10.1016/j.athoracsur.2013.04.073. Epub 2013 Jun 26. PMID: 23809730. CrossRef Pubmed
7. Dohy Z, Szabo L, Toth A, Czimbalmos C, Horvath R, Horvath V, Suhai FI, Geller L, Merkely B, Vago H. Prognostic significance of cardiac magnetic resonance-based markers in patients with hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2021 Jun;37(6):2027-2036. doi: 10.1007/s10554-021-02165-8. Epub 2021 Feb 8. PMID: 33555536; PMCID: PMC8255255. CrossRef Pubmed
8. Noureldin RA, Liu S, Nacif MS, Judge DP, Halushka MK, Abraham TP, Ho C, Bluemke DA. The diagnosis of hypertrophic cardiomyopathy by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012 Feb 20;14(1):17. doi: 10.1186/1532-429X-14-17. PMID: 22348519; PMCID: PMC3309929. CrossRef Pubmed
9. Green JJ, Berger JS, Kramer CM, Salerno M. Prognostic value of late gadolinium enhancement in clinical outcomes for hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2012 Apr;5(4):370-7. doi: 10.1016/j.jcmg.2011.11.021. PMID: 22498326. CrossRef Pubmed
10. Wang J, Zhang Z, Li Y, Xu Y, Wan K, Chen Y. Variable and Limited Predictive Value of the European Society of Cardiology Hypertrophic Cardiomyopathy Sudden-Death Risk Model: A Meta-analysis. Can J Cardiol. 2019 Dec;35(12):1791-1799. doi: 10.1016/j.cjca.2019.05.004. Epub 2019 May 10. PMID: 31474312. CrossRef Pubmed
11. Rowin EJ, Maron MS. The Role of Cardiac MRI in the Diagnosis and Risk Stratification of Hypertrophic Cardiomyopathy. Arrhythm Electrophysiol Rev. 2016;5(3):197-202. doi:10.15420/aer.2016:13:3. CrossRef Pubmed
12. Pelliccia F, Gersh BJ, Camici PG. Gaps in Evidence for Risk Stratification for Sudden Cardiac Death in Hypertrophic Cardiomyopathy. Circulation. 2021 Jan 12;143(2):101-103. doi: 10.1161/CIRCULATIONAHA.120.051968. Epub 2021 Jan 11. PMID: 33428428. CrossRef Pubmed
Copyright Information

