Review Article
Mar 2023, 32:1
First online: 6 April 2023
Review Article
Review Article: Cardiac Arrhythmia among Hospitalized COVID-19 Patients
Kevin Wibawa,1 Kintan Sari Nastiti,1 Siti Annisaa Meiviani,1 Pangeran Akbar Syah,2 Suhendiwijaya Suhendiwijaya,2 Yandi Ariffudin2
2 Cardiologist, Gunung Jati General Hospital, Cirebon, West Java, Indonesia.
Main and corresponding author: Kevin Wibawa.
Email: kevin.wibawa24@gmail.com, ORCID number 0000-0003-2975-1900
ABSTRACT
Cardiac arrhythmia is one of the common complications among hospitalized COVID-19 patients. The incidence of arrhythmia in COVID-19 varies from 5.9% to 16.7%. This literature review to explore the epidemiology, risk factors, clinical manifestation, pathophysiology, outcomes, and management of hospitalized COVID -19 patients with cardiac arrhythmia. The literature search and review of the literature was performed on PubMed and Google Scholar from January 2020 to July 2021.
Age, comorbidities, and COVID-19 disease severity may increase the risk to develop arrhythmia. Hypertension, coronary artery disease, heart failure, diabetes mellitus, and renal disease are more frequently observed patients with arrhythmia. The proposed pathophysiology of arrhythmia in COVID-19 are myocardial injury, hypoxia, cytokine storm, and drugs side effects. In addition, comorbidity, pre-existing scar or conduction defect, history of previous arrhythmia, electrolyte abnormalities may play a role in the pathophysiology of tachyarrhythmia and bradyarrhythmia. The in-hospital mortality, need of intensive care unit, need of mechanical ventilation or non-invasive ventilation, hypotension, and thromboembolic event were higher in hospitalized COVID-19 patients with arrhythmia. The general managements were to treat the underlying COVID-19 infection and to tackle the hemodynamic disturbances due to tachyarrhythmia or bradyarrhythmia.
Cardiac arrhythmia is a common complication among hospitalized COVID-19 patients. Hospitalized COVID-19 patients with tachyarrhythmia or bradyarrhythmia had worse in-hospital outcomes compared with patients without arrhythmia.
BACKGROUND
Coronavirus disease 2019 (COVID-19), first identified in Wuhan during December 2019, has various complications, including cardiac arrhythmia.1, 2 There are no specific symptoms regarding arrhythmia in COVID-19. Patients may present with symptoms related to tachy- or bradyarrhythmia.3 The presentation of cardiac arrhythmia varies from sinus tachycardia to malignant ventricular arrhythmia (VA) or complete heart block (CHB).4-7
The incidence of cardiac arrhythmia among hospitalized COVID-19 patients varies from 5.9% to 16.7%.8, 9 In addition, patients with more severe forms of disease may have a higher risk of developing cardiac arrhythmia. The same study from Wuhan reported 44.4% hospitalized COVID-19 patients in the ICU had cardiac arrhythmia.8 Atrial fibrillation (AF) was the most common tachyarrhythmia (21%) reported in hospitalized COVID-19 patients. The reported incidence varies from 5.6% to 21%.10, 11 Sinus bradycardia and complete heart block were also reported in 8% hospitalized COVID-19 patients.10
Despite the high incidence of cardiac arrhythmia among hospitalized COVID-19 patients, there is a paucity and variability regarding the exact nature and outcome of hospitalized COVID-19 patients with cardiac arrhythmia. Direct viral infection to the heart was commonly reported as the pathophysiology of arrhythmia among COVID-19 patients. However, other potential mechanisms are also described among many studies.4, 12-15 Furthermore, patient comorbidities and certain drugs that were used to treat COVID-19 may be involved in the mechanism of arrhythmia in COVID-19.14, 15 Therefore, we performed this literature review to explore the epidemiology, risk factors, clinical manifestation, pathophysiology, outcomes, and management of hospitalized COVID-19 patients with cardiac arrhythmia.
MAIN TEXT
Data Sources
The literature search was performed on PubMed and Google Scholar from January 2020 to July 2021 and updated on 3rd February 2023. We included the search terms: “arrhythmia” OR “supraventricular tachycardia” OR “atrial fibrillation” OR “atrial flutter” OR “atrioventricular nodal re-entry tachycardia” OR “atrioventricular re-entry tachycardia” OR “sinus tachycardia” OR “ventricular arrhythmia” OR “ventricular tachycardia” OR “ventricular fibrillation” OR “premature ventricular complex” OR “premature atrial complex” OR “sinus bradycardia” OR “atrioventricular block” OR “sinus pause” OR “sinus arrest” AND “COVID-19” OR “coronavirus disease 2019” OR “coronavirus” AND “hospitalization” OR “hospitalized”. The inclusion criteria were: 1) Confirmed COVID-19 patients with polymerase chain reaction 2) Age ≥18 years old; 3) Studies reported the outcomes of confirmed COVID-19 patients with arrhythmia. Search was limited only to English language manuscripts. Citations in each included article during the main search were reviewed for potential relevance.
Epidemiology
The early study from Wuhan reported the incidence of arrhythmia among hospitalized COVID-19 patients was 16.7% and may reach as high as 44.4% in patients admitted to the ICU.7 This discrepancy in arrhythmia incidence between non-ICU and ICU population was due to the high population of ICU patients in the study. To date, many studies have reported various incidences of arrhythmia. Tachyarrhythmia was more commonly reported compared with bradyarrhythmia. However, the true incidence of tachyarrhythmia and bradyarrhythmia may be underestimated since not every study used continuous electrocardiography (ECG) monitoring for every hospitalized patient.16-30
Tachyarrhythmia can manifest as sinus tachycardia, atrial fibrillation (AF), atrial flutter (AFL), atrial tachycardia, and atrioventricular nodal re-entrant tachycardia (AVNRT).10, 24 Sinus tachycardia was reported in 24.44-64.8% from hospitalized COVID-19 patients.11, 18, 25 Atrial fibrillation /Atrial flutter was reported in 4%-15.8% hospitalized patients.19, 24, 31 The incidence of pre-existing AF, new-onset AF, and new-onset AFL were 9.64%, 6.63% and 0.77%, respectively.16, 17 Atrial tachycardia was reported 0.2% hospitalized patients.22 On the other hand, the incidence of AVNRT remains scarce since patients with AVNRT are typically younger and lack significant comorbidities.32
The incidence of Ventricular Tachycardia (VT)/Ventricular Fibrillation (VF) varied from 1.28%-17.5%.11, 17, 18, 21 The reported incidence of VT/VF was higher in patients with elevated troponin level compared to normal troponin level, 17.3% and 1.5% respectively.33
The data regarding incidence of bradyarrhythmia is limited.11, 12, 17, 18, 22, 34-38 Sinus bradycardia, relative sinus bradycardia, and atrioventricular (AV) block was reported among hospitalized COVID-19 patients.17, 34, 35, 37, 38 Sinus bradycardia and relative bradycardia may occur in 4.9% and 36% of hospitalized COVID-19 patients.11, 37 Severe bradyarrhythmia such as high grade AV block and complete heart block (CHB) was also reported during admission or during hospitalization.11, 16, 34 The incidence of high grade AV block and CHB was 0.26% and 0.26-1.4% respectively.11, 16 A study also reported 15 patients with 2:1 AV block and CHB (1 with 2:1 AV block; 14 with CHB) in which 13 out of 15 patients presented with CHB during initial admission.34
Clinical presentation
The clinical presentations of tachyarrhythmias and bradyarrhythmias among COVID-19 patients are the same as the usual presentations. The clinical presentations are summarized in Table 1. To date, there is no single specific pattern for arrhythmia in SARS-CoV-2 infection.39 Respiratory symptoms such as fever, altered sensorium, and cough may also present during initial admission to the hospital.16, 34 In tachyarrhythmia, palpitations and/or syncope was commonly reported.16 In bradyarrhythmia patients presenting with high grade AV block or CHB, syncope was the most common symptom.34 Less common symptoms such as fatigue and dyspnea are quite common in patients with high grade AV block or CHB.34
TABLE 1. AGE, SYMPTOMS, AND REPORTED COMORBIDITIES
Comorbidities and risk factor of developing arrhythmia
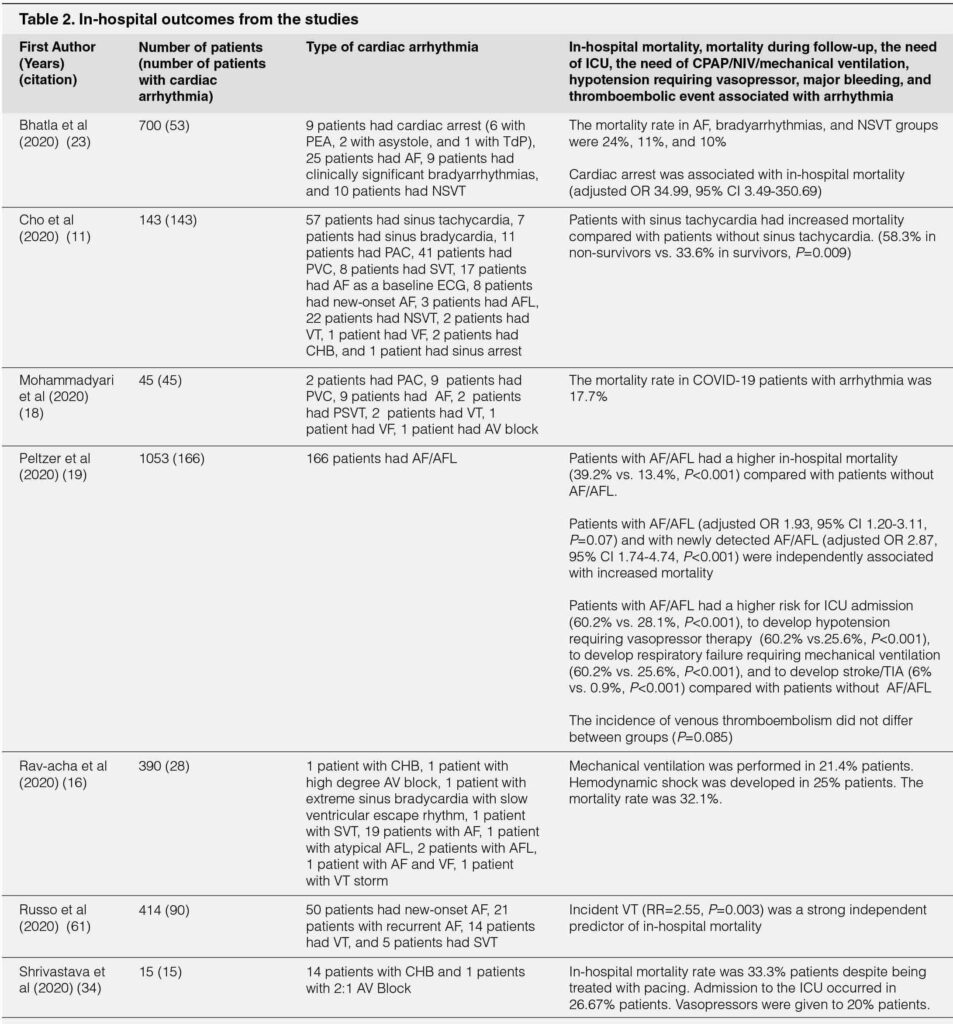
The age and comorbidities were summarized in Table 1. Patients with arrhythmias were older and had more comorbidities than those without.16, 17, 19, 22, 40 Age was significantly associated with incident arrhythmia during hospitalization.17, 40 An increase in 1 year of age was independently associated with new-onset AF (adjusted OR 1.05;95% CI 1.02-1.09).23 Hypertension, diabetes mellitus, coronary artery disease, heart failure, renal disease, prior stroke, past arrhythmia, and prior history of AF were more commonly observed in patients with arrhythmia compared with patients without arrhythmia.16, 17, 19, 22, 23
There were some risk factors associated with new-onset arrhythmia among COVID-19 patients. One study reported disease severity was associated with higher probability to develop new-onset arrhythmia.16 Patients with moderate, severe, and critical disease severity had a higher probability to develop new-onset arrhythmia compared with mild severity, with OR of 4.81, 8.86, and 15.79 respectively.16 Another study also reported cardiovascular disease (OR 3.307,;95% CI 1.329-8.232;P=0.01) was significantly associated with incident arrhythmia in COVID-19.17 The presence of heart failure (HF) as comorbidity was associated with new-onset tachyarrhythmia (OR 4.78;95% CI 1.31-17.48;P=0.018)16 and was associated with bradyarrhythmia (adjusted OR 9.75;95% CI 1.95-48.65).23
Biomarker of Cytokine Storm and Myocardial Injury
During cytokine storms, the level of pro-inflammatory cytokines, such as interleukin-6 (IL-6), interleukin-2 (IL-2), and tumour necrosis factor-α (TNFα) are markedly elevated.4, 41 Also, the level of inflammatory markers such as c-reactive protein (CRP), lactate dehydrogenase (LDH), and ferritin are markedly elevated.40, 42 Indeed, hospitalized patients with arrhythmia have significantly higher IL-6, LDH, CRP, and ferritin levels compared with hospitalized patients without arrhythmia.17, 19, 22 Interestingly, patients with four times elevated level of IL-6 had a higher chance of developing atrial arrhythmias compared with patients with less than two times elevated level of IL-6 (P=0.05).22
Other than elevated cytokines and inflammatory markers, the level biomarkers of myocardial injury were significantly higher in patients with arrhythmia than those without arrhythmia.11, 17, 19, 22 Patients with arrhythmia had significantly elevated level of high-sensitivity troponin T (hsTnT), high-sensitivity troponin I (hsTnI), creatinine kinase-MB fraction (CK-MB), brain natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NTproBNP).17, 19, 22 Specific arrhythmia, such as new-onset AF, AFL, and NSVT was more common in patients with elevated troponin levels.11
Proposed pathophysiology of cardiac arrhythmia
The COVID-19 has 3 phases of clinical evolution, but the phases can overlap each other. The first is a mild phase occurring in the first 7 days of infection indicated by constitutional symptoms with about 80% cases are resolved.39, 41 SARS-CoV-2 enters the target cell by using angiotensin converting enzyme 2 (ACE2) receptor. ACE2 receptor is expressed on the small intestine, kidney, heart, epicardial adipose tissue, pericytes, blood vessel, and lung.43-46 Direct myocardial injury may occur in this stage.47 If the infection is worsening, the patient will develop the second phase or moderate pneumonia. Approximately, 15% patients will develop the second stage or moderate pneumonia. In this phase, acute lung injury may cause hypoxemia and oxygen demand-supply mismatch in which further development of anaerobic metabolic will eventually start metabolic derangements.4, 41, 47 Finally, the third phase or severe pneumonia occurs in approximately 5% patients.41 This phase is marked by cytokine storm and is associated with severe systemic inflammation and multiorgan failure.47 The level of pro-inflammatory cytokines (IL-6, IL-2, and TNFα) and inflammatory markers (CRP, LDH, ferritin) are markedly elevated.4, 41 Myocardial injury, which happened in 12% of COVID-19 patients with or without cardiovascular comorbidity,41 may cause arrhythmia exacerbation as explained in Figure 1. The injury can disrupt cardiac electrical conduction system.48 However, troponin level was not elevated in every COVID-19 patient with arrhythmia, suggesting other mechanism may be involved during the arrhythmia occurrence.4, 15, 49
Hypoxia due to ARDS in COVID-19 infection may cause electrolyte imbalance and further precipitate arrhythmia. Hypoxia activates anaerobic glycolysis, reduces intracellular pH, and increases cytosolic calcium levels. Moreover, increased cytosolic calcium levels can facilitate early and late depolarisations, as well as temporal alterations in the action potential duration.4 Furthermore, hypoxia reduces the rapid delayed rectifier potassium (IKr), resulting in the increased extracellular potassium levels.7, 50 This can decrease the threshold for depolarization and accelerates electrical conduction.50 On the other hand, hypoxia can increase the number of late sodium current (INaL) along with IKr reduction can prolong ventricular repolarization which lead into re-entrant arrhythmia.7, 50
Cytokine storm may increase the risk of developing arrhythmia. 4, 47 Cytokine storms affect potassium and calcium channels, which may prolong ventricular action potential.3 Pro-inflammatory cytokine such as IL-6 can cause hyperactivation of cardiac sympathetic system and trigger malignant arrhythmia in patients with long QT interval.50 Furthermore, IL-6 is thought to be the central mediator of pro-inflammatory cytokine and organizes its responses from immune cells.48 Higher viral load expressed higher levels of pro-inflammatory cytokines.15 Side effects of certain drugs may also trigger cardiac arrhythmia in COVID-19 patients.4 Hydroxychloroquine and azithromycin are known for their effects in prolonging QT interval by inhibiting the IKr.3 The net effect of QT prolongation to the IKr by multiple QT prolonging drugs is synergistic.51 Therefore, special attention must be put on patients receiving multiple drugs that can block IKr, such as those who receive chloroquine or hydroxychloroquine combined with other antiviral drugs (lopinavir/ritonavir) or even antibiotics (macrolides and fluoroquinolones).51, 52 In addition, COVID-19 patients may have prolonged QT interval due to hypokalemia or hypomagnesemia precipitated by either diarrhea or diuretic agents.15 Furthermore, IL-6 inhibits cytochrome P450 (CYP) 3A4, which in turn increases the bioavailability of QTprolonging drugs.15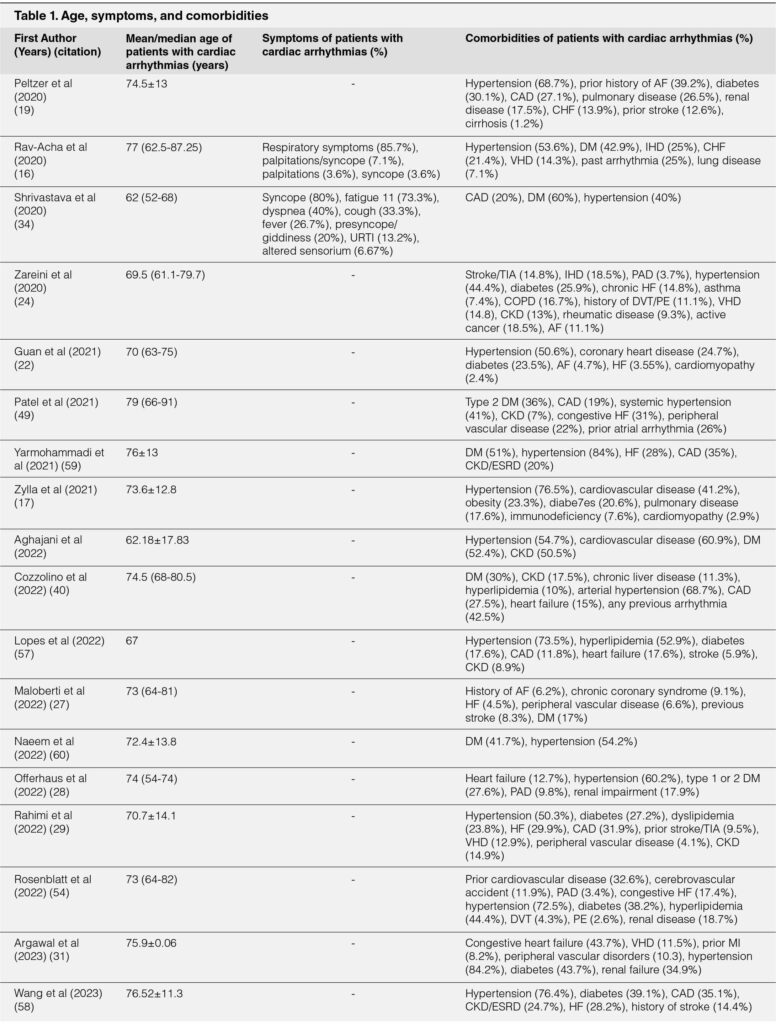
FIGURE 1. PROPOSED PATHOPHYSIOLOGY OF CARDIAC ARRHYTHMIA
Proposed pathophysiology of tachyarrhythmia
Multiple mechanisms are involved in the pathophysiology of tachyarrhythmia in COVID-19. Myocardial injury, renal failure, electrolyte abnormalities, cytokine storm, and use of certain drugs may trigger tachyarrhythmia.9, 10, 15, 28, 45, 53
Supraventricular tachycardia (SVT) in COVID-19 can manifest as sinus tachycardia, AF, AFL, atrial tachycardia, and AVNRT.3, 10, 11, 32 The presence of sinus tachycardia, AF, and AFL in COVID-19 was frequently reported. However, AVNRT is uncommon due to population in studies consist of mainly younger age and lack significant comorbidities.32
The development of sinus tachycardia in COVID-19 patients represent acute course of illness that usually cause by hypovolemia, hypoperfusion, hypoxia, fever, and fear/anxiety during COVID-19.15, 32, 53 Meanwhile, AF which is the second most common type of supraventricular arrhythmia after sinus tachycardia occur during hospitalization (new-onset, recurrence of previous dysrhythmia, or AF with persisting rapid ventricular response) and post recovery.10, 32, 46 In most cases, new onset AF in COVID-19 patients occurred in the older population and with at least 1 pre-existing risk factor.28, 45, 54 The incubation period of COVID-19 is relatively short and is not enough to develop fibrosis as a substrate for AF. Therefore, the patients may have had a pre-existing substrate and acute COVID-19 infection triggered the new onset AF.45, 54 Other factor such as pro-inflammatory cytokine for instance TNF-α may increase risk of AF vulnerability by triggering pulmonary cardiomyocyte vein activity that results in increased activity of sodium-calcium exchanger (NCX) and impaired sarcoplasmic reticulum (SR) ATPase.9
Malignant arrhythmia, defined as rapid ventricular tachycardia lasting >30s, inducing hemodynamic instability, or ventricular fibrillation, occurred in 17.3% patients with myocardial injury.15 Factors that may precipitate this process including the elevation of troponin and CRP level, hyperadrenergic state in the scarring myocardium, and inflammation.15, 41, 48 Myocardial inflammation is reported to have the potential in prolonging ventricular action potential duration, cause severe necrosis, and create a re-entry area that triggers VT and VF.15, 48 Myocardial inflammation may persist in approximately 60% patients who recently recovered from COVID-19 infection, irrespective of pre-existing condition, severity of acute COVID-19 infection, and the course of COVID-19 infection.55
Proposed pathophysiology of bradyarrhythmia
Bradyarrhythmia are less commonly reported in hospitalized COVID-19 patients.34-36, 38, 43, 56 It may manifest as sinus bradycardia, relative bradycardia, AV block, high grade AV block, or CHB. New onset bradycardia during hospitalization may be a marker of worsening cytokine storm.36, 53
Various mechanisms are involved in the pathophysiology of bradyarrhythmia.38, 56 Myocardial injury may cause transient AV block because of the affected electrical conduction system.41 This was supported by the presence of SARS-CoV-2 RNA in cardiac myocytes and endothelial cells from autopsies of COVID-19 patients.34, 56 In addition, severe hypoxia, inflamed cardiac pacemaker cells, worsening of pre-existing conduction disease, reaction to the systemic inflammation, and inappropriate drug response may also trigger bradyarrhythmia.34, 36, 38, 49 However, bradyarrhythmia due to reversible causes, e.g. in patients with AV block related to acute coronary syndrome and metabolic abnormalities, are less likely induced by COVID-19.21 Pro-inflammatory cytokines such as IL-6 is highly associated with relative bradycardia and may directly supress Sinoatrial (SA) Node, increase vagal tone, and reduce heart rate variability.36, 43 Increasing level of CRP and ferritin was also reported to have association with CHB and high grade AV block.34
Electrocardiographic monitoring, QT prolonging drugs, and QTc interval
Hospitalized COVID-19 patients may develop arrhythmia at the time of hospital admission or during hospitalization period.11, 16-30, 34, 57 Continuous electrocardiographic (ECG) monitoring for every hospitalized COVID-19 patient is required to evaluate arrhythmic events.24 ECG monitoring is also performed during brady- and tachyarrhythmia therapy.48 Other than that, ECG monitoring provides QT corrected (QTc) interval measurement.39
The QTc interval >450 milliseconds in males and >460 milliseconds in females can be defined as prolonged QTc interval. Lead II or V5 is the preferable lead to measure QTc interval and bazett’s correction is the most commonly used formula to calculate the QTc interval.53 Sometimes, the combination of QT prolonging drugs is inevitable. Since the QT prolongation effect is synergistic,51 the combination of azithromycin, chloroquine, or hydroxychloroquine can prolong QTc interval and may induce arrhythmia.36, 39, 43 The combination of dual antiviral therapy was independent predictors of QT prolongation (OR 12.46;95% CI 2.09-74.20;P<0.01).20
Ideally, monitoring of QTc interval in patients receiving QT prolonging drugs is mandatory, especially in patients admitted to the ICU. 53 But, single-lead electrocardiography (ECG) devices tend to underestimate the QTc interval. Twelve-lead ECG devices provide more accurate and reliable QT interval measurement compared with single-lead ECG,39 however this situation puts a considerable strain on medical personnel during the COVID-19 pandemic. Therefore, contactless monitoring and telemetry systems may be preferred and act as an alternative. Smart watches, smartphones, and smart beds can provide wireless monitoring for in-hospital QTc interval monitoring.53
In-hospital outcomes
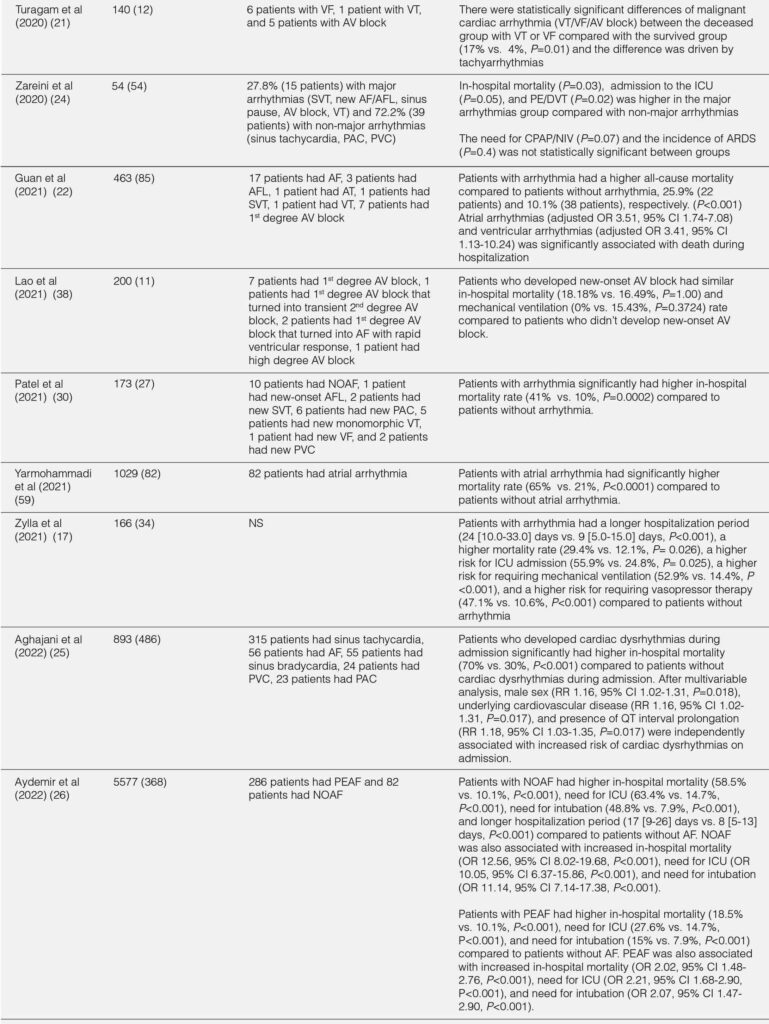
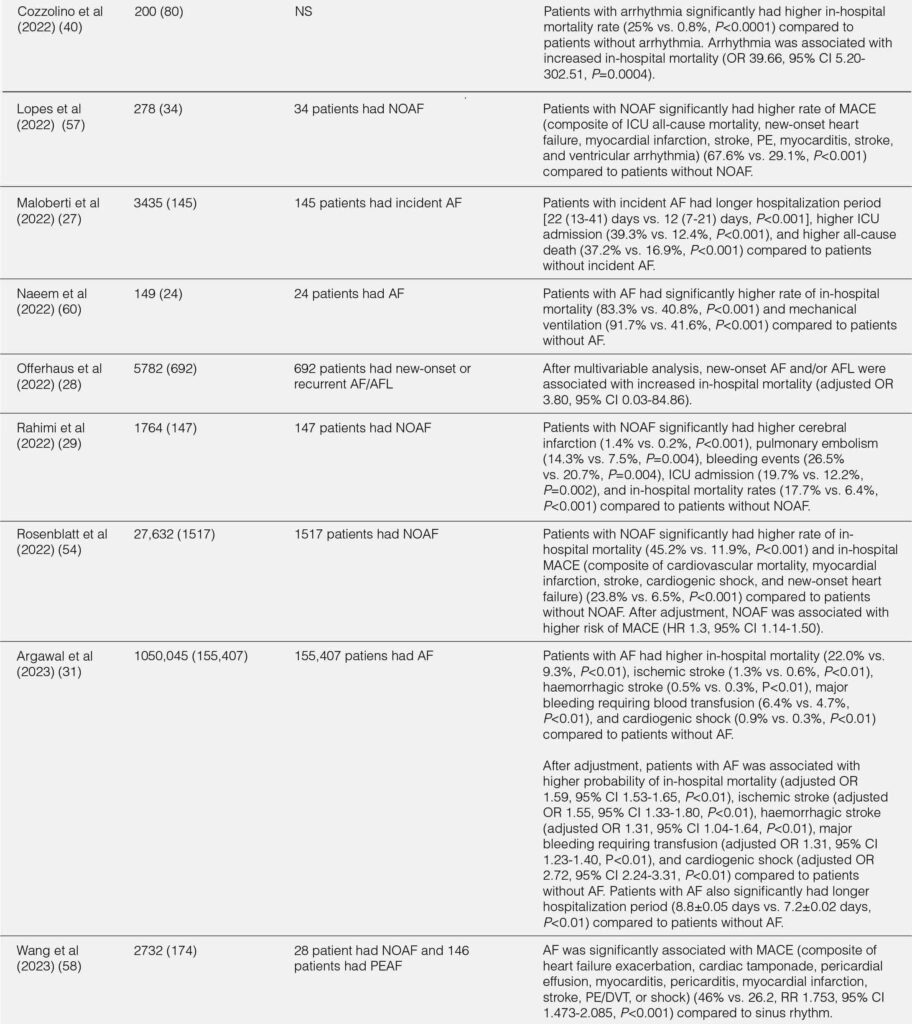
The outcomes of hospitalized COVID-19 patients with arrhythmia were summarized in Table 2. Overall, hospitalized COVID-19 patients with arrhythmia had worse outcomes compared with hospitalized COVID-19 patients without arrhythmia.17, 19, 22, 40 The overall duration of hospitalization and duration of hospitalization in the ICU/intermediate care unit (IMC) was longer in hospitalized COVID-19 patients.17 Patients with arrhythmia had a higher rate of ICU or IMC admission (OR 2.37;95% CI 1.10-5.09;P=0.03), mechanical ventilation due to severe respiratory failure (OR 6.69;95% CI 2.92-15.35;P<0.001), vasopressors (47.1% vs. 10.6%;P<0.001), and high-flow nasal cannula (HFNC) / noninvasive ventilation (NIV) (41.2% vs. 19.1%;P=0.01) compared with patients without arrhythmia.17 In terms of cardiac events, patients with arrhythmia were more likely to develop myocardial infarction (8.8% vs. 0.8%;P=0.021) or heart failure (34.12% vs. 8.73%;P<0.001) during hospitalization compared with patients without arrhythmia.17, 22 The in-hospital mortality was significantly higher in patients with arrhythmia during hospitalization than patients without arrhythmia (OR 3.02;95% CI 1.22-7.46;P=0.02).17 In addition, patients with arrhythmia during initial presentation had increased risk of death compared with patients without arrhythmia (P<0.001).22 Furthermore, elevated troponin level was associated with higher in-hospital mortality compared with normal troponin level (34.8 vs. 16.7%;P=0.014).11
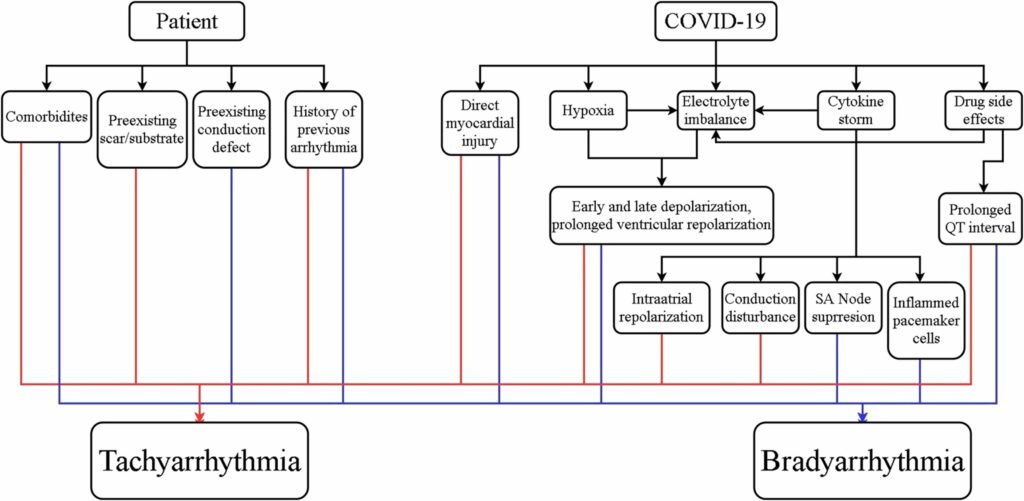
TABLE 2. IN-HOSPITAL OUTCOMES FROM THE STUDIES
Association between type of arrhythmia and in-hospital outcome
Sinus tachycardia, the most common tachycardia, was associated with increased in-hospital mortality (58.3% in nonsurvivors vs. 33.6% in survivors; P=0.009).11 In AF/AFL, the in-hospital mortality was significantly higher in patients with AF/AFL compared with patients without AF/AFL.19, 28 Also, both new onset and pre-existing AF were associated with higher rate of in hospital mortality, need of ICU, need for mechanical ventilation, and shock compared to patients without AF.26, 57-60 Compared to non-major arrhythmias (sinus tachycardia, premature atrial complexes, premature ventricular complexes), patients with major arrhythmia (SVT, new AF/AFL, sinus pause, and VT) had higher in-hospital mortality rate and higher rate incidence of pulmonary embolism/deep vein thrombosis.24 Furthermore, VT significantly increased the risk of in-hospital mortality (RR=2.55;P=0.003).61
Bradyarrhythmia has been considered with worse outcome and more severe disease compared with tachyarrhythmia.43 The in-hospital mortality of bradyarrhythmia in hospitalized COVID-19 patients was 18.8% to 57%, despite being treated with pacemaker.34, 35, 38 Self-reverted rhythm back to sinus was reported in patients with CHB with narrow and wide complex escape rhythm.34 Moreover, broad QRS complex escape rhythm may predict worse inflammatory state and poorer rhythm recovery compared with narrow QRS complex escape rhythm.34
Management of arrhythmia from real world data
Several studies reported real world data regarding the management of arrhythmia in hospitalized COVID-19 patients.16, 17, 19-21, 34-37, 56, 62 The general approach is to treat the underlying COVID-19 infection and is to treat hemodynamic disturbances due to tachyarrhythmia or bradyarrhythmia. Antiviral, antibiotic, and supportive treatment can be administered for COVID-19.8, 20 Caution must be used regarding the combination of antiviral and antibiotic that may result in QTc prolongation.20 In addition, lopinavir and ritonavir can cause PR prolongation, QRS widening, and QT prolongation.52 Hemodynamic disturbances in form of hypotension and desaturation can be treated with inotropes and non-invasive or invasive ventilation.16, 21, 34The management of tachyarrhythmia in COVID-19 from available data mostly followed the recommendation from advanced cardiac life support (ACLS) algorithm. Electrical cardioversion can be performed in AF with hemodynamic instability.17, 57, 63 Pharmacological cardioversion using amiodarone can be administered to convert AF, AFL, and AT to sinus rhythm.17 Intravenous beta blocker may be administered to treat NSVT.20 In case of pulseless VT, torsades des pointes (TdP), or VF, the management was performed according to ACLS algorithm.20, 64 One compelling finding was the anticoagulant treatment to prevent stroke or transient ischemic attack (TIA) in COVID-19 with AF or AFL. Therapeutic or prophylaxis dose of low-molecular weight heparin (LMWH) or non-vitamin K oral anticoagulant (NOAC) can be administered as anticoagulant treatment in hospitalized COVID-19 patients with AF or AFL.17, 19 Approximately, 80% of stroke or TIA occurred in hospitalized COVID-19 with AF or AFL who were not on therapeutic dose of anticoagulant.19 Therefore, therapeutic dose of anticoagulant may be preferred over prophylaxis dose of anticoagulant in COVID-19 with new-onset AF without contraindication to anticoagulant treatment.19
In COVID-19 with bradycardia, the initial treatment also followed the recommendation from ACLS algorithm. There were several reports of sinus bradycardia and relative bradycardia related to COVID-19 infection and no specific treatment was reported.36, 37 Temporary pacemaker may be required to treat hemodynamic disturbances due to sinus pause, sinus sick syndrome, or high grade or CHB.34, 35, 56 Self-reverted from narrow complex escape rhythm in high grade or CHB to sinus rhythm was reported in 42.9% patients.34 In contrast, no self-reverted from wide complex escape rhythm to sinus rhythm was reported and permanent pacemaker was implanted.34 The need of permanent pacemaker due to sinus pause and high grade or CHB was between 46.7% and 57.1%.34, 35 Permanent pacemaker implantation can be performed after clinical recovery or 14 days after COVID-19 infection.34 Asystole and pulseless electrical activity (PEA) can be treated according to the ACLS algorithm.20, 64
CONCLUSIONS
Based on this literature review, cardiac arrhythmia is a common complication in COVID-19 and the true incidence is not fully appreciated. To date, there were no specific symptoms or signs to alert the physician regarding the presence of tachyarrhythmia or bradyarrhythmia. The occurrence of arrhythmia among hospitalized COVID-19 patients may be predictor of worse inhospital outcomes. Furthermore, larger studies are needed to fully understand the exact nature of arrhythmia in COVID-19 and to understand the effect of arrhythmia in COVID-19 in relation to in-hospital outcomes.
Keywords:
arrhythmia, COVID-19, supraventricular arrhythmia, ventricular arrhythmia
DECLARATIONS
Funding
Not applicable
Competing interests
The authors declare that they have no competing interests
Consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and materials
Not applicable
Code availability
Not applicable
Authors’ contributions
All authors have read and approved the manuscript
Kevin Wibawa: conception of the manuscript, drafted the manuscript, circulated for the
review, revised the final manuscript, read and approved the final manuscript
Kintan Sari Nastiti: drafted the manuscript and circulated for the review, read and approved the final manuscript
Siti Annisaa Meiviani: drafted the manuscript and circulated for the review, read and approved the final manuscript
Pangeran Akbar Syah: reviewed and revising the manuscript, read and approved the final manuscript
Suhendiwijaya Suhendiwijaya: reviewed and revising the manuscript, Suhendiwijaya read and approved the final manuscript
Yandi Ariffudin: reviewed and revising the manuscript, read and approved the final manuscript
REFERENCES
1. Pollard CA, Morran MP, Nestor-Kalinoski AL. The COVID-19 pandemic: a global health crisis. Physiol Genomics. 2020;52(11):549-557. doi:10.1152/physiolgenomics.00089.2020 CrossRef Pubmed
2. Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31(5):1003-1008. doi:10.1111/jce.14479 CrossRef Pubmed
3. Desai AD, Boursiquot BC, Melki L, Wan EY. Management of Arrhythmias Associated with COVID-19. Curr Cardiol Rep. 2020;23(1):2. doi:10.1007/s11886-020-01434-7 CrossRef Pubmed
4. Dherange P, Lang J, Qian P, et al. Arrhythmias and COVID-19. JACC Clin Electrophysiol. 2020;6(9):1193-1204. doi:10.1016/j.jacep.2020.08.002 CrossRef Pubmed
5. Kuck K-H. Arrhythmias and sudden cardiac death in the COVID-19 pandemic. Herz. 2020;45(4):325-326. doi:10.1007/s00059-020-04924-0 CrossRef Pubmed
6. Kochav Stephanie M., Coromilas Ellie, Nalbandian Ani, et al. Cardiac Arrhythmias in COVID-19 Infection. Circulation: Arrhythmia and Electrophysiology. 2020;13(6):e008719. doi:10.1161/CIRCEP.120.008719 CrossRef Pubmed
7. Wang Y, Wang Z, Tse G, et al. Cardiac arrhythmias in patients with COVID-19. Journal of Arrhythmia. 2020;36(5):827-836. doi:10.1002/joa3.12405 CrossRef Pubmed
8. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069. doi:10.1001/jama.2020.1585 CrossRef Pubmed
9. Hu Y-F, Cheng W-H, Hung Y, et al. Management of Atrial Fibrillation in COVID-19 Pandemic. Circ J. 2020;84(10):1679-1685. doi:10.1253/circj.CJ-20-0566 CrossRef Pubmed
10. Gopinathannair R, Merchant FM, Lakkireddy DR, et al. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020;59(2):329-336. doi:10.1007/s10840-020-00789-9 CrossRef Pubmed
11. Cho JH, Namazi A, Shelton R, et al. Cardiac arrhythmias in hospitalized patients with COVID-19: A prospective observational study in the western United States. PLOS ONE 2020;15(12):e0244533. doi:10.1371/journal.pone.0244533 CrossRef Pubmed
12. Babapoor-Farrokhran S, Rasekhi RT, Gill D, Babapoor S, Amanullah A. Arrhythmia in COVID-19. SN Compr Clin Med. 2020:1-6. doi:10.1007/s42399-020-00454-2 CrossRef Pubmed
13. Crotti L, Arbelo E. COVID-19 treatments, QT interval, and arrhythmic risk: The need for an international registry on arrhythmias. Heart Rhythm. 2020;17(9):1423-1424. doi:10.1016/j.hrthm.2020.05.024 CrossRef Pubmed
14. Lazzerini PE, Boutjdir M, Capecchi PL. COVID-19, Arrhythmic Risk, and Inflammation: Mind the Gap! Circulation. 2020;142(1):7-9. doi:10.1161/CIRCULATIONAHA.120.047293 CrossRef Pubmed
15. Giustino G, Pinney SP, Lala A, et al. Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia: JACC Focus Seminar. J Am Coll Cardiol. 2020;76(17):2011-2023. doi:10.1016/j.jacc.2020.08.059 CrossRef Pubmed
16. Rav-Acha M, Orlev A, Itzhaki I, et al. Cardiac arrhythmias amongst hospitalised Coronavirus 2019 (COVID-19) patients: Prevalence, characterisation, and clinical algorithm to classify arrhythmic risk. International Journal of Clinical Practice. 2021;75(4):e13788. doi:10.1111/ijcp.13788 CrossRef Pubmed
17. Zylla MM, Merle U, Vey JA, et al. Predictors and Prognostic Implications of Cardiac Arrhythmias in Patients Hospitalized for COVID-19. J Clin Med. 2021;10(1). doi:10.3390/jcm10010133 CrossRef Pubmed
18. Mohammadyari E, Ahmadi I, Mohammadyari A, Tavan H, Norozi S. The frequency of arrhythmias in COVID-19 patients, a study in the Shahid Mostafa Khomeini Hospital of Ilam from March to August 2020. New Microbes and New Infections. 2021. doi:10.1016/j.nmni.2021.100867 CrossRef Pubmed
19. Peltzer B, Manocha KK, Ying X, et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophysiol. 2020. doi:10.1111/jce.14770 CrossRef Pubmed
20. Santoro F, Monitillo F, Raimondo P, et al. QTc interval prolongation and life-threatening arrhythmias during hospitalization in patients with COVID-19. Results from a multi-center prospective registry. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1578 CrossRef Pubmed
21. Turagam Mohit K., Musikantow Daniel, Goldman Martin E., et al. Malignant Arrhythmias in Patients With COVID-19. Circulation: Arrhythmia and Electrophysiology. 2020;13(11):e008920. doi:10.1161/CIRCEP.120.008920 CrossRef Pubmed
22. Guan H, Liu J, Ding J, et al. Arrhythmias in patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: Incidences and implications. J Electrocardiol. 2021;65:96-101. doi:10.1016/j.jelectrocard.2021.01.012 CrossRef Pubmed
23. Bhatla A, Mayer MM, Adusumalli S, et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439-1444. doi:10.1016/j.hrthm.2020.06.016 CrossRef Pubmed
24. Zareini B, Rajan D, El-Sheikh M, et al. Cardiac arrhythmias in patients hospitalized with COVID-19: The ACOVID study. Heart Rhythm O2. 2021;2(3):304-308. doi:10.1016/j.hroo.2021.03.008 CrossRef Pubmed
25. Aghajani MH, Haghighi M, Sistanizad M, et al. Cardiac dysrhythmia in COVID-19 patients; occurrence and risk factors: a retrospective cohort study. Front Emerg Med. Published online May 8, 2022. doi:10.18502/fem.v6i3.9399 CrossRef
26. Aydemir S, Aksakal E, Aydınyılmaz F, et al. Does new onset and preexisting atrial fibrillation predict mortality in COVID-19 patients? Egypt Heart J. 2022;74(1):53. doi:10.1186/s43044-022-00291-9 CrossRef Pubmed
27. Maloberti A, Giannattasio C, Rebora P, et al. Incident Atrial Fibrillation and In-Hospital Mortality in SARS-CoV-2 Patients. Biomedicines. 2022;10(8):1940. doi:10.3390/biomedicines10081940 CrossRef Pubmed
28. Offerhaus JA, Joosten LPT, van Smeden M, et al. Sex- and age specific association of new-onset atrial fibrillation with in-hospital mortality in hospitalised COVID-19 patients. IJC Heart Vasc. 2022;39:100970. doi:10.1016/j.ijcha.2022.100970 CrossRef Pubmed
29. Rahimi FS, Afaghi S, Tarki FE, Omran HS, Nasirpour MH. Risk factors, thromboembolic events, and clinical course of New-Onset Atrial Fibrillation among COVID-19 hospitalized patients: A multicenter cross-sectional analysis in Iran. Health Sci Rep. 2022;5(6):e813. doi:10.1002/hsr2.813 CrossRef Pubmed
30. Patel NH, Rutland J, Tecson KM. Arrhythmias and Intraventricular Conduction Disturbances in Patients Hospitalized With Coronavirus Disease 2019. Am J Cardiol. 2022;162:111-115. doi:10.1016/j.amjcard.2021.08.052 CrossRef Pubmed
31. Agarwal S, Munir MB, Stavrakis S, Piccini JP, Asad ZUA. The impact of atrial fibrillation on outcomes in patients hospitalized with COVID-19. Eur J Intern Med. 2023;0(0). doi:10.1016/j.ejim.2023.01.017 CrossRef Pubmed
32. Long B, Brady WJ, Bridwell RE, et al. Electrocardiographic manifestations of COVID-19. Am J Emerg Med. 2021;41:96-103. doi:10.1016/j.ajem.2020.12.060 CrossRef Pubmed
33. Guo T, Fan Y, Chen M, et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811-818. doi:10.1001/jamacardio.2020.1017 CrossRef Pubmed
34. Shrivastava A, Pandit BN, Thakur AK, Nath RK, Aggarwal P. Epidemiological, demographic, laboratory, clinical management, and outcome data of symptomatic bradyarrhythmia in COVID-19 patients. Cir Cardiov. 2021;28(3):144-150. doi:10.1016/j.circv.2021.01.008 CrossRef Pubmed
35. Chinitz JS, Goyal R, Harding M, et al. Brady-arrhythmias in Patients with COVID-19: Marker of Poor Prognosis? Pacing Clin Electrophysiol. 2020. doi:10.1111/pace.14042 CrossRef Pubmed
36. Amaratunga EA, Corwin DS, Moran L, Snyder R. Bradycardia in Patients With COVID-19: A Calm Before the Storm? Cureus. 2020;12(6):e8599. doi:10.7759/cureus.8599 CrossRef Pubmed
37. Capoferri G, Osthoff M, Egli A, Stoeckle M, Bassetti S. Relative bradycardia in patients with COVID-19. Clinical Microbiology and Infection. 2021;27(2):295-296. doi:10.1016/j.cmi.2020.08.013 CrossRef Pubmed
38. Lao N, Lim J, Bashir H, et al. Incidence of Atrioventricular Blocks and its Association with In-Hospital Mortality and Morbidity in Patients with Coronavirus Disease 2019. J Cardiol. 2022;79(4):482-488. doi:10.1016/j.jjcc.2021.10.025 CrossRef Pubmed
39. Martínez-Rubio A, Ascoeta S, Taibi F, Guindo Soldevila J. Coronavirus Disease 2019 and Cardiac Arrhythmias. Eur Cardiol. 2020;15:e66. doi:10.15420/ecr.2020.23 CrossRef Pubmed
40. Cozzolino D, Romano C, Nevola R, et al. COVID-19 and arrhythmia: The factors associated and the role of myocardial electrical impulse propagation. An observational study based on cardiac telemetric monitoring. Front Cardiovasc Med. 2022;9:912474. doi:10.3389/fcvm.2022.912474 CrossRef Pubmed
41. Azevedo RB, Botelho BG, Hollanda JVG de, et al. Covid-19 and the cardiovascular system: a comprehensive review. J Hum Hypertens. 2021;35(1):4-11. doi:10.1038/s41371-020-0387-4 CrossRef Pubmed
42. Mostaghim A, Sinha P, Bielick C, et al. Clinical outcomes and inflammatory marker levels in patients with Covid-19 and obesity at an inner-city safety net hospital. PLOS ONE. 2020;15(12):e0243888. doi:10.1371/journal.pone.0243888 CrossRef Pubmed
43. Douedi S, Mararenko A, Alshami A, et al. COVID-19 induced bradyarrhythmia and relative bradycardia: An overview. Journal of Arrhythmia. 2021. doi:10.1002/joa3.12578 CrossRef Pubmed
44. Furqan MM, Verma BR, Cremer PC, Imazio M, Klein AL. Pericardial Diseases in COVID19: a Contemporary Review. Curr Cardiol Rep. 2021;23(7):90. doi:10.1007/s11886-021-01519-x CrossRef Pubmed
45. Gawałko M, Kapłon-Cieślicka A, Hohl M, Dobrev D, Linz D. COVID-19 associated atrial fibrillation: Incidence, putative mechanisms and potential clinical implications. Int J Cardiol Heart Vasc. 2020;30:100631. doi:10.1016/j.ijcha.2020.100631 CrossRef Pubmed
46. Stone E, Kiat H, McLachlan CS. Atrial fibrillation in COVID-19: A review of possible mechanisms. FASEB J. 2020;34(9):11347-11354. doi:10.1096/fj.202001613 CrossRef Pubmed
47. Taqi H, Farooqui A, Adlan A, Davis G. COVID-19: Cardiac arrhythmias. Heart (British Cardiac Society). 2020:15.
48. Siripanthong B, Nazarian S, Muser D, et al. Recognizing COVID-19-related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463-1471. doi:10.1016/j.hrthm.2020.05.001 CrossRef Pubmed
49. Patel NH, Rutland J, Tecson KM. Arrhythmias and Intraventricular Conduction Disturbances in Patients Hospitalized With Coronavirus Disease 2019. Am J Cardiol. 2022;162:111-115. doi:10.1016/j.amjcard.2021.08.052 CrossRef Pubmed
50. Yadav R, Bansal R, Budakoty S, Barwad P. COVID-19 and sudden cardiac death: A new potential risk. Indian Heart Journal. 2020;72(5):333-336. doi:10.1016/j.ihj.2020.10.001 CrossRef Pubmed
51. Carpenter A, Chambers OJ, El Harchi A, et al. COVID-19 Management and Arrhythmia: Risks and Challenges for Clinicians Treating Patients Affected by SARS-CoV-2. Frontiers in Cardiovascular Medicine. 2020;7:85. doi:10.3389/fcvm.2020.00085 CrossRef Pubmed
52. Ridjab DA, Ivan I, Budiman F, Juliawati DJ. Current evidence for the risk of PR prolongation, QRS widening, QT prolongation, from lopinavir, ritonavir, atazanavir, and saquinavir. Medicine (Baltimore). 2021;100(31):e26787. doi:10.1097/MD.0000000000026787 CrossRef Pubmed
53. Manolis AS, Manolis AA, Manolis TA, Apostolopoulos EJ, Papatheou D, Melita H. COVID-19 infection and cardiac arrhythmias. Trends Cardiovasc Med. 2020;30(8):451-460. doi:10.1016/j.tcm.2020.08.002 CrossRef Pubmed
54. Rosenblatt AG, Ayers CR, Rao A, et al. New-Onset Atrial Fibrillation in Patients Hospitalized With COVID-19: Results From the American Heart Association COVID-19 Cardiovascular Registry. Circ Arrhythm Electrophysiol. 2022;15(5):e010666. doi:10.1161/CIRCEP.121.010666 CrossRef Pubmed
55. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiology. 2020;5(11):1265-1273. doi:10.1001/jamacardio.2020.3557 CrossRef Pubmed
56. Gupta MD, Qamar A, Mp G, et al. Bradyarrhythmias in patients with COVID-19: A case series. Indian Pacing Electrophysiol J. 2020;20(5):211-212. doi:10.1016/j.ipej.2020.08.004 CrossRef Pubmed
57. Lopes V, Baptista, Joao Pedro, Moreira, Nadia, Martins, Paulo, Goncalves, Lino. Association Between New-Onset Atrial Fibrillation and Cardiovascular Outcomes in Critically Ill COVID-19 Patients. J Atr Fibrillation. 2022;15(6):20200632. doi:10.4022/jafib.20200632
58. Wang L, Hoang L, Aten K, et al. Mortality and Major Adverse Cardiovascular Events in Hospitalized Patients With Atrial Fibrillation With COVID-19. Am J Cardiol. 2023;189:41-48. doi:10.1016/j.amjcard.2022.11.040 CrossRef Pubmed
59. Yarmohammadi H, Morrow JP, Dizon J, et al. Frequency of Atrial Arrhythmia in Hospitalized Patients With COVID-19. Am J Cardiol. 2021;147:52-57. doi:10.1016/j.amjcard.2021.01.039 CrossRef Pubmed
60. Naeem KB, Ali OA, Alsiddig E, Osman I, Alhajiri A, Abdulrazzaq N. New-Onset Atrial Fibrillation Independently Predicts In-Hospital Mortality In Critically Ill COVID-19 Patients Admitted To The Intensive Care Unit. 2022;4(1).
61. Russo V, Maio MD, Mottola FF, et al. Clinical characteristics and prognosis of hospitalized COVID-19 patients with incident sustained tachyarrhythmias: A multicenter observational study. European Journal of Clinical Investigation. 2020;50(12):e13387. doi:10.1111/eci.13387 CrossRef Pubmed
62. Gavin W, Campbell E, Zaidi S-A, et al. Clinical characteristics, outcomes and prognosticators in adult patients hospitalized with COVID-19. Am J Infect Control. 2021;49(2):158-165. doi:10.1016/j.ajic.2020.07.005 CrossRef Pubmed
63. Colon CM, Barrios JG, Chiles JW, et al. Atrial Arrhythmias in COVID-19 Patients. JACC Clin Electrophysiol. 2020;6(9):1189-1190. doi:10.1016/j.jacep.2020.05.015 CrossRef Pubmed
64. Abrams MP, Coromilas EJ, Wan EY, et al. Malignant ventricular arrhythmias in patients with severe acute respiratory distress syndrome due to COVID-19 without significant structural heart disease. HeartRhythm Case Rep. 2020;6(11):858-862. doi:10.1016/j.hrcr.2020.08.017 CrossRef Pubmed
Copyright Information
