Case Report
1 March 2024, 33:1
First online: March 2024
Case Report
STEMI and Thyroid Storm: A Case Report of Thyrotoxicosis-Induced Cardiomyopathy
Billy Joseph David,1 Bea Christine Joyce Buot,1 Rod Castro1
1 Department of Medicine, St. Luke’s Medical Center – Quezon City, Philippines
ABSTRACT
Thyrotoxicosis-induced cardiomyopathy is a rare but potentially fatal form of non-ischemic cardiomyopathy that warrants immediate medical intervention. A 51-year-old female with no known disease sought consultation because of severe chest pain accompanied by easy fatigability and palpitation. Upon arrival at the ED, there was persistence of chest pain accompanied by vomiting, tachycardia and hypotension. ECG showed STEMI in the lead aVR, and she eventually underwent coronary angiogram, which revealed normal coronary arteries. Ancillaries showed severe hyperthyroidism with a Burch – Wartofsky score of 50. There was an elevated NT-proBNP level, and echocardiogram showed a reduced EF of 28.7% with dilated LV and global hypokinesia. She was hooked to a norepinephrine and dobutamine drip and immediately initiated a hyperthyroid regimen, specifically methimazole, propranolol, and steroids. She was admitted to the critical care unit where initiation of SGLT-2 inhibitor and spironolactone were administered as part of the heart failure regimen. When she was out of thyroid storm, a sestamibi myocardial perfusion scan was requested, which showed normal results with improved EF. The goals of management in thyrotoxicosis-induced cardiomyopathy are early initiation of heart failure regimen, medication to prevent further ventricular remodeling, and treatment of thyroid storm. Early recognition and prompt administration of hyperthyroid treatment leads to a decreased burden of the patient’s disease and a good overall prognosis.
Key words
STEMI, Thyrotoxicosis-induced cardiomyopathy, Heart Failure,
Thyroid Storm, Graves’ disease
INTRODUCTION
Thyrotoxicosis is caused by excess thyroid hormone levels from inappropriately high circulating hormone concentrations that can cause multi-organ dysfunction. One of the well-known cardiovascular manifestations includes stress cardiomyopathy, coronary vasospasm, myocarditis, and even mimic ST-segment elevation myocardial infarction (STEMI).1 Thyrotoxicosis and hyperthyroidism are known to cause high-output failure and left ventricular hypertrophy due to sustained increases in both cardiac output and preload from increased expression of myocardial sarcoplasmic reticulum calcium-dependent adenosine triphosphate (ATP), which results in increased heart rate and myocardial contractility.2-3 Untreated hyperthyroidism may result in STEMI, which is rarely encountered as its first clinical manifestation.4
CASE DESCRIPTION
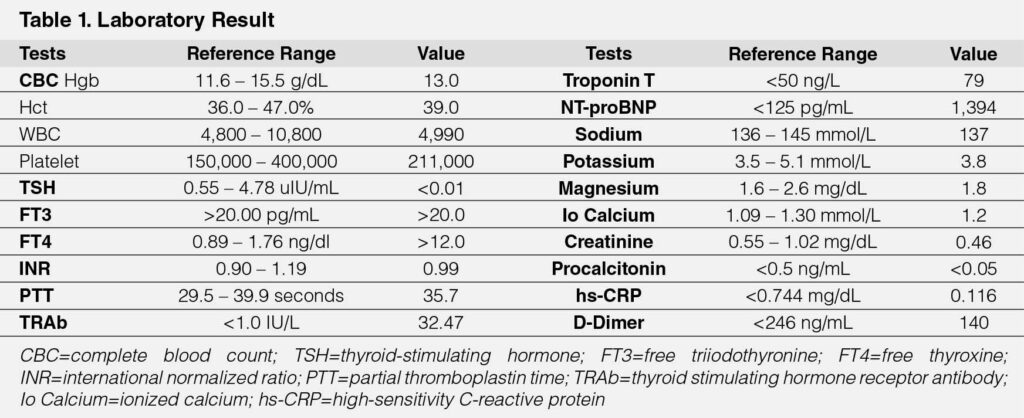
A 51-year-old female with no known disease sought consultation because of sudden onset severe chest pain accompanied by one week history of easy fatigability, palpitations, and three pillow orthopnea. Upon arrival at the ED, there was persistent chest pain characterized as continuous, sharp, and nonradiating accompanied by vomiting, tachycardia as high as 140 beats per minute (BPM), and hypotension as low as 70/50 mmHg in both arms. There was no carotid bruit with a jugular venous pressure of 8 mmHg above the sternal angle. On cardiac examination, there were distinct S1 and S2 sounds, no heaves, thrills, murmurs, or S3 gallop. Pulses were full and equal in all extremities with no bipedal edema. The patient was initially administered fluid resuscitation of 1 L of crystalloid solution, hooked to a low-dose norepinephrine drip, and given analgesia using morphine. Blood pressure was stabilized; however, she was still tachycardic; hence, esmolol IV was administered. The electrocardiogram (ECG) showed STEMI in lead aVR with ST depression on leads V2–V6 (Figure 1A) with an elevated troponin T of 79 ng/L. She was also started on an acute coronary syndrome (ACS) regimen, namely aspirin 300mg, ticagrelor 180mg, enoxaparin 60 mg, and rosuvastatin 40 mg. Coronary angiogram revealed normal coronary arteries (Figure 1B). She was eventually admitted to the cardiology critical care unit. Ancillaries were performed to determine the etiology of the disease (See Table 1). These tests showed severe hyperthyroidism with TSH of <0.01 uIU/mL, FT4 >12 ng/dL and FT3 >20 pg/mL with Burch Wartofsky score of 50. Neck ultrasound showed diffuse parenchymal enlargement of the thyroid gland with TSH receptor antibodies (TRAb) of 32.47 IU/L, suggestive of Graves’ disease. Inflammatory markers, specifically high-sensitivity C-reactive protein (hs-CRP) and D-dimer, were normal. There was elevated NT-proBNP (1394 pg/mL), and echocardiogram showed a reduced EF of 28.7% with dilated LV and global hypokinesia with mild pulmonary hypertension. There was an increasing norepinephrine requirement; hence, started on dobutamine drip. There was immediate initiation of hyperthyroid regimen, specifically methimazole, propranolol, and steroids. Vasopressors were eventually titrated off. The patient was also managed as heart failure with reduced ejection fraction; hence, guided directed medical therapy was started by administering sacubitril-valsartan, empagliflozin, and spironolactone. The patient was closely monitored during critical care and was eventually transferred to a regular room. When she was out of thyroid storm, additional ancillaries were performed, specifically myocardial perfusion imaging using technetium-99m sestamibi scan, which showed no scintigraphic evidence of resting ischemia with markedly improved LV systolic function with ejection fraction of 61% (Figure 1C). She was eventually discharged with methimazole, propranolol, and the pillars of heart failure therapy.
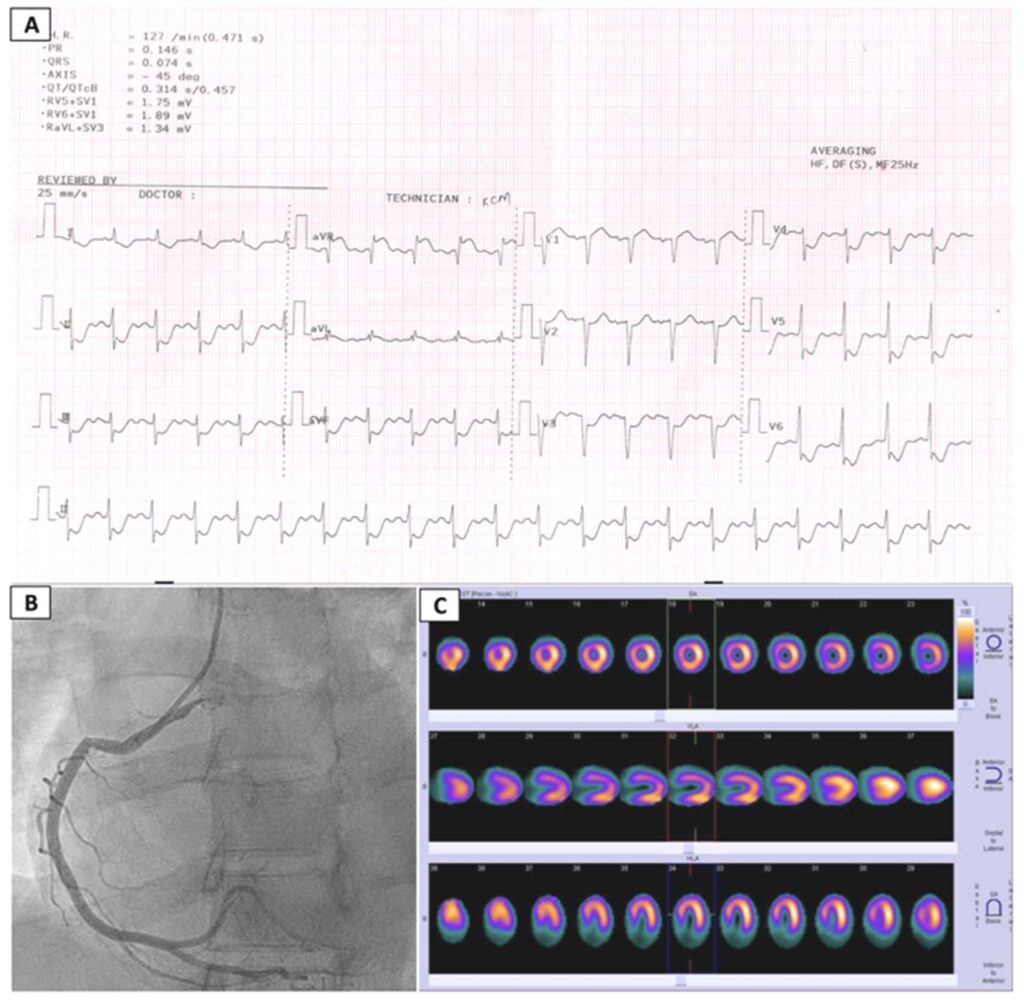
Figure 1: Diagnostic work-up
A) ECG showing ST-segment elevation in lead aVR and ST segment depression in leads V2-V6
B) Coronary angiogram revealing normal coronary arteries
C) Sestamibi scan showing no scintigraphic evidence of resting ischemia
DISCUSSION
A clear connection exists between thyrotoxicosis and heart conditions, notably atrial fibrillation and tachycardia-induced cardiomyopathy. Coronary vasospasm should be suspected among patients presenting with signs and symptoms of acute myocardial infarction in the setting of a hyperthyroid state but with normal coronary arteries on angiography. In relation to our patient’s case, there were no localized ST elevations that may be due to coronary vasospasm or acute coronary thrombus formation. Another important differential to consider is stress cardiomyopathy because it is prevalent in this specific age group with similar presenting symptoms. Pertinent triggers causing stress cardiomyopathy are physical or emotional stress that causes excessive activation of the sympathetic nervous system, resulting in increased catecholamine production and endothelial injury. The classical morphological pattern of LV regional wall motion abnormality is apical hypokinesia, akinesia, or dyskinesia (apical ballooning) with basal hyperkinesia.5 Our patient presented with transient systolic dysfunction and elevated cardiac enzymes that were consistent with stress cardiomyopathy. In addition, thyrotoxicosis causes excessive sympathetic stimulation, which is believed to be the underlying pathophysiological mechanism of stress cardiomyopathy.However, the patient did not present with the characteristic wall-motion abnormalities and localized ST elevation commonly observed in stress cardiomyopathy. Another pertinent differential to consider is autoimmune myocarditis, which is a complication of Grave’s disease. The pathophysiology of myocarditis associated with Graves’ disease is unclear, but the presence of thyrotropin receptor in cardiac tissue has been demonstrated by reverse transcriptase polymerase chain reaction, suggesting a possible mechanism for stimulation by thyrotropin receptor antibodies.6
In this case, the patient had ST elevation in the lead avR with diffuse ST depression on ECG with global hypokinesia on echocardiogram with normal coronary arteries on coronary angiogram, which is highly suggestive of autoimmune myocarditis. Hence, immediate intervention to address the cause of myocarditis is vital, and early initiation of guideline-directed medical treatment for heart failure decreases the risk of dilated cardiomyopathy and ventricular remodeling.
CONCLUSION
Thyrotoxicosis can result in cardiovascular complications, including STEMI. Early recognition and a thorough diagnostic workup are essential to effectively manage this condition. The initial evaluation typically involves a combination of clinical assessment, electrocardiography (ECG), echocardiography, and laboratory tests. Ischemic evaluation by myocardial perfusion scan or coronary angiogram will be beneficial in arriving at the correct diagnosis. The goals of management in thyrotoxicosis-induced cardiomyopathy are immediate treatment of thyroid storm, early initiation of heart failure regimen, and medication to prevent further ventricular remodeling. Prompt administration of hyperthyroid treatment leads to a decreased burden of the patient’s disease and a good overall prognosis.
ACKNOWLEDGMENTS
None
REFERENCES
1. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001 Feb 15;344(7):501-9. doi: 10.1056/NEJM200102153440707. PMID: 11172193. CrossRef Pubmed
2. Sharma A, Stan MN: Thyrotoxicosis: diagnosis and management. Mayo Clin Proc. 2019, 94:1048-64. 10.1016/j.mayocp.2018.10.011 CrossRef Pubmed
3. Khalil Y, Dube MD, Woods L. Thyrotoxicosis-Induced Cardiomyopathy With Systolic Dysfunction. Cureus. 2023 Jan 20;15(1):e33988. doi: 10.7759/cureus.33988. PMID: 36694856; PMCID: PMC9858885. CrossRef Pubmed
4. Osuna PM, Udovcic M, Sharma MD. Hyperthyroidism and the Heart. Methodist Debakey Cardiovasc J. 2017 Apr-Jun;13(2):60-63. doi: 10.14797/mdcj-13-2-60. PMID: 28740583; PMCID: PMC5512680. CrossRef Pubmed
5. Al Houri HN, Jomaa S, Jabra M, et al. Pathophysiology of stress cardiomyopathy: A comprehensive literature review. Ann Med Surg (Lond). 2022 Sep 15;82:104671. doi: 10.1016/j.amsu.2022.104671. PMID: 36268377 CrossRef Pubmed
6. Koshiyama H, Sellitti DF, Akamizu T, Doi SQ, Takeuchi Y, Inoue D, Sakaguchi H, Takemura G, Sato Y, Takatsu Y, Nakao K. Cardiomyopathy associated with Graves’ disease. Clin Endocrinol (Oxf). 1996 Jul;45(1):111-6. PMID: 8796147. Pubmed
Copyright Information
