Expert Review
Mar 2023, 32:1
First online: 27 March 2023
Expert Review
PART 1: RAAS Blockers – Are They All The Same?
Vijaya Bharath Ramasamy1
Email: vijaya.bharath@islandhospital.com
INTRODUCTION
The renin-angiotensin-aldosterone system (RAAS) is a crucial mechanism by which our bodies maintain blood pressure and fluid & electrolyte balance. Any disruption to this system may upset the carefully maintained equilibrium, leading to development of hypertension and cardiovascular (CV) diseases.
Thus, RAAS has been a key therapeutic target in hypertension since the serendipity discovery of angiotensin converting enzyme inhibitors (ACEis) in the 1970s. The use of ACEis have also evolved over the decades, from a stand-alone blood pressure (BP) lowering agent to critical underpinnings of CV protection in high-risk patients following improved elucidation of its role.
ACEis CONFERS BROAD CV PROTECTION
Captopril, the first ACEi, was introduced into routine clinical practice back in 1981. However, it took another half a dozen years before the first randomized clinical trial (RCT) to investigate the effects of ACEis in severe heart failure – the CONSENSUS study was published.1 Although it was a small study with 253 subjects, it was momentous nevertheless as it showed patients randomized to enalapril had a massive 40% reduction in mortality at 6 months compared with placebo. This sparked great optimism in the potential for ACEis to save lives in those with established CV diseases, and at the same fueled intense research into the value of ACEi amongst patients with CV risks but without an established disease.
The first major study however to demonstrate conclusive benefits of using ACEis in heart failure was the SOLVD trial in 1991.2 In this study, treatment with enalapril over the course of slightly more than three years prevented around 50 premature deaths and an additional 350 hospitalizations for every 1000 patients. In the years to follow, ACEis firmly cemented its place as part of standard care for the treatment of heart failure, with evidence from subsequent trials like SAVE and V-HeFT II.3, 4
Before long, its use was extended to patients post myocardial infarction (MI). In the AIRE and TRACE placebo-controlled study,patients assigned to ramipril and trandolapril within a few days of MI had a subsequent 27% and 22% relative risk reduction in mortality respectively.5, 6 In addition, patients in the trandolapril treatment arm in the TRACE study had a 29% lesser chance of progressing to severe heart failure. In what remains as still the largest RCT involving an ACEi, the ISIS-4 study, over 58000 patients were randomized to either captopril or placebo following suspected acute MI.7 Captopril treatment was associated with almost 5 fewer deaths for every 1000 patients treated for just a month.
CV PROTECTION IN LOWER RISK PATIENTS
By the late 1990s, the protective role of ACEis in heart failure and in post MI became indisputable. Nonetheless, it still remained ambiguous whether or not this degree of protection could be replicated in a relatively lower risk group of patients who may have multiple CV risk factors, but are otherwise stable with no evidence of heart failure or recent acute MI.
At the turn of the century, in what will become a landmark CV trial, the HOPE study reported its findings.8 More than 9000 patients who are 55 years or older with either stable CV disease or diabetes plus one other CV risk factor, were randomised to ramipril treatment or placebo arm for over 5 years. Despite only a very minimal BP reductions of 3/2 mm Hg, the implications were far reaching – with 26% reductions in death from CV causes, 20% reductions in MI, 32% lower stroke incidences and a 23% reduction in heart failure presentations in the treatment arm.
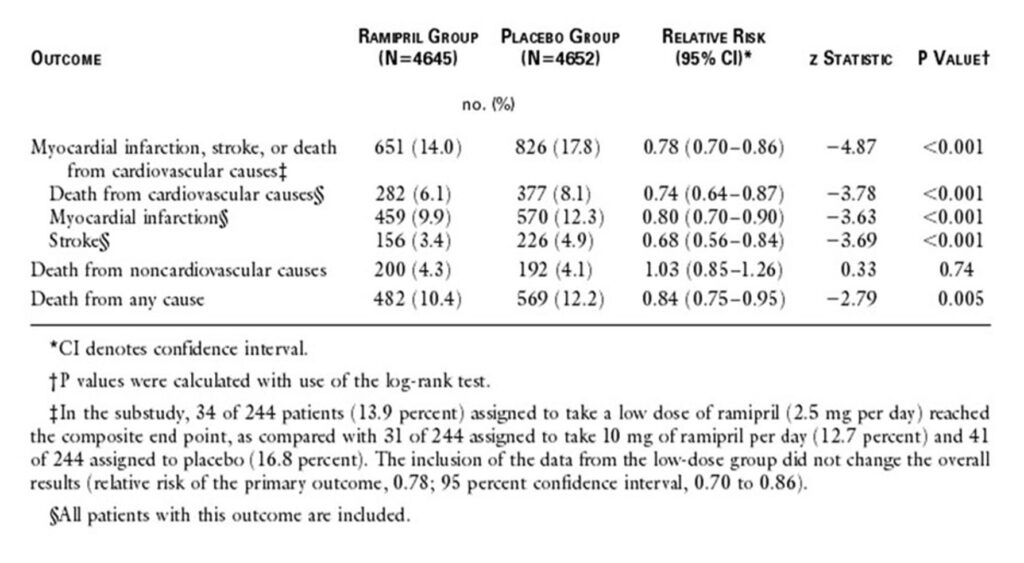
The subsequent EUROPA study was fundamentally very much alike the HOPE trial, in which patients with stable CV disease were randomised to either perindopril or placebo.9 However, the EUROPA study intended to examine the effectiveness of using ACEis in an even more lower risk group of patients. Not only EUROPA study recruited much younger patients than the HOPE study, it also recruited substantially lesser hypertensives and diabetic patients than the HOPE study. Despite this and notwithstanding a modest BP reductions of 5/2 mm Hg during the double-blinded treatment, patients taking perindopril had a 22% reduction in non-fatal MI compared to placebo. With these two trials, ACEis were now no longer drugs merely to lower BP or to treat cardiac remodeling following acute heart failure or MI, they are now therapy to prevent them.
Intriguingly, the first study to investigate the role of an ACEi in hypertension had to wait until 1999 when the CAPPP study was published – 18 years after ACEi became available.10 Since then large-scale studies have reported their evaluations via ANBP 2 trial, HYVET trial and the ASCOT study. ANBP 2 trial demonstrated superiority of enalapril over hydrochlorothiazide in CV outcomes, including death, despite no significant differences in BP.11 Meanwhile, the HYVET trial showed in patients aged 80 years and above, treatment with indapamide plus perindopril demonstrated huge reductions in all cause mortality, CV death, and incidences of stroke & heart failure, compared with placebo.12 Last but not least, in the landmark ASCOT study, where patients were randomized to an atenolol plus bendroflumethiazide or amlodipine plus perindopril treatment, subjects in the latter treatment arm had significantly lesser adverse CV events and induced less diabetes than the former despite almost exact blood pressure reductions in both arms.13
Finally, another group of patients who have derived significant benefits from ACEis are those with diabetes and diabetic nephropathy. The first suggestion that ACEis may help in this population was gathered from the HOPE study, in which in addition to the CV benefits discussed above, patients receiving ramipril also had a 16% reduction in diabetes related complications.8 This stimulated further examinations into this new discovery, with a substudy of the main HOPE study, called the MICRO HOPE study reporting even more striking findings when only the diabetic populations were analyzed.14 Whilst the parent HOPE study showed a reduction of 26% in CV death in the ramipril treatment arm, the reduction became even more pronounced at 37% when only patients with diabetes examined in the MIRCO HOPE study. In addition, overt nephropathy was reduced by 24% in the same study in patients receiving ramipril. This renal protective effect was also replicated amongst type 1 diabetic patients with nephropathy in a pivotal study involving 409 patients who were randomized to captopril versus placebo.15 Treatment with captopril for a median of 3 years showed a staggering 50% relative risk reduction in combined endpoints of death, dialysis and transplantation, with the protective effects more marked than what could be expected with BP lowering effects alone. Not only were ACEis fast becoming a therapy for nephropathy and to reduce adverse CV events amongst patients with diabetes, it also showed in subsequent studies to be able to prevent nephropathy in diabetic patients with no proteinuria. For example, in the ADVANCE trial, patients subjected to treatment with fixed combination of perindopril and indapamide showed 21% reduction in new microalbuminuria compared to placebo.16 All these beneficial findings eventually culminated in ACEis becoming the first choice antihypertensive agent in patients with diabetes.19
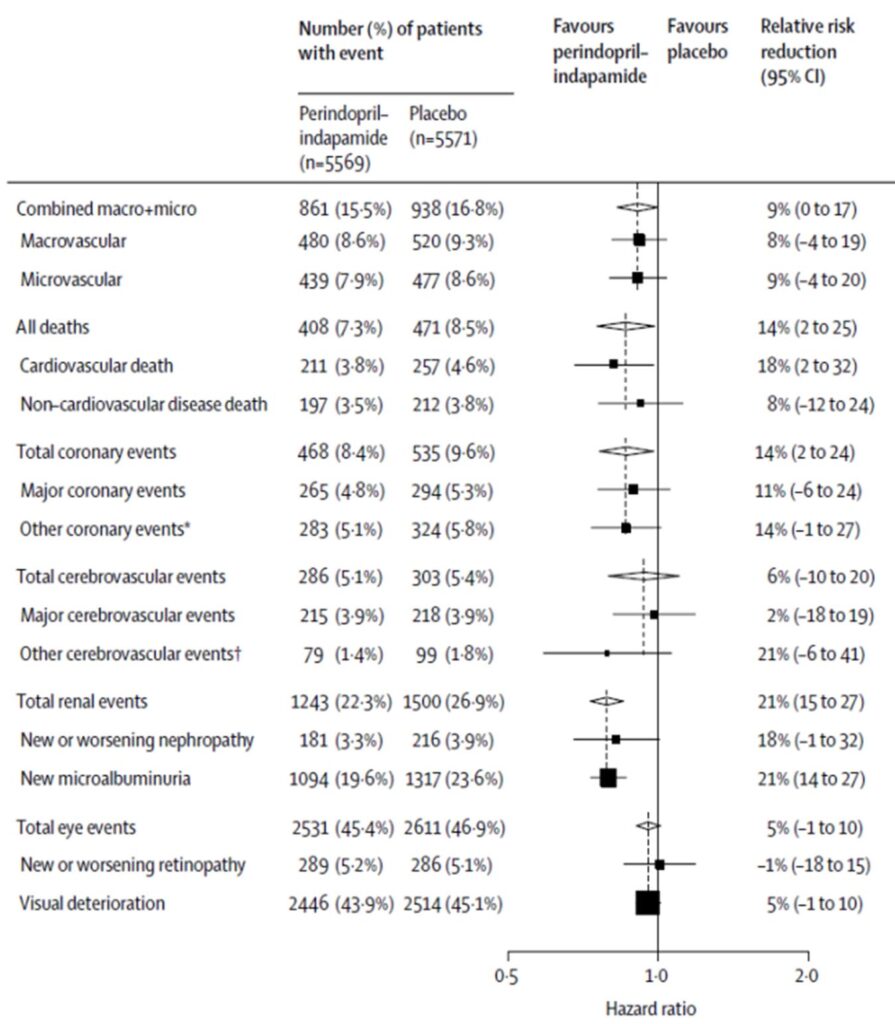
* Other coronary events=unstable angina requiring hospitalisation, coronary revascularisation or silent myocardial infarction. †Other cerebrovascular events=transient ischaemic attack (including amaurosis fugax) or subarachnoid haemorrhage. Black squares=point estimates (with area proportional to number of events);
horizontal lines=95% CI. Diamonds=point estimate and 95% CI for overall effects. Vertical broken lines=point estimates for overall effect, within categories.
* ADVANCE study consisted of 11140 patients with type 2 diabetes who were randomised to treatment with a fixed combination of perindopril and indapamide or matching placebo, in addition to current therapy. The
primary endpoints were composites of major macrovascular and microvascular events, defined as death from cardiovascular disease, non-fatal stroke or non-fatal myocardial
ACEis: CV BENEFITS INDEPENDENT OF BP LOWERING
Although it is long been suspected by the proponents of ACEis that its CV benefits are independent of its BP lowering, the challenge is however to extricate this variable from the eventual outcome to conclusively prove this notion. In the above-mentioned landmark trials of HOPE and EUROPA, the significant CV risk reductions seen with ACEis were in spite of very minimal BP reductions observed in the treatment group – between 3/2mm Hg to 5/2mm Hg.8, 9 Such small BP reductions are usually not expected to produce substantial CV events reduction, let alone in trials where the mean baseline BP of these trials above are not at a level to be defined as hypertension (between 133/79 mm Hg – 139/78 mm Hg).
However, more convincing evidences come from studies that shows when ACEis were compared to other antihypertensive agents, ACEis can reduce adverse CV events, particularly MI, despite similar degree of BP reductions. For example, in the earlier briefly mentioned ANBP 2 trial, patients who were randomized to ACEis had statistically significant reductions in MI compared to those assigned to diuretics despite exactly similar BP reductions of 26/12 mm Hg in both the groups at 5 years.11 Again, in the AASK trial, patients who were randomized to ramipril had slower decline in glomerular filtration rate (GFR), decrease in urinary protein excretion, and delayed onset of end stage renal disease (ESRD) or death, as compared to amlodipine at 3 years.18 This beneficial effect occurred in the presence of similar levels of BP control in both groups.
In a meta-analysis involving nearly 180000 patients, a metaregression examination to delineate the effects of different variables were performed and it was shown quite evidently that ACEis reduced the incidence of MI by 12% on its own, when the BP reduction variable was isolated.17
The overall benefits conferred by ACEis in patients with hypertension, diabetes & nephropathy, CV risk factors, heart failure and post MI, in addition to its BP lowering effect, has increasingly led to its appearances in guidelines for use as part of first line treatment for these indications.19-23 Angiotensin 2 receptor blockers (ARBs), appearing in the scene years later, benefitted from the widespread belief that the CV benefits observed with ACEis will also be duplicated with its use as they both block the RAAS after all. But after over two decades of ARB use, coupled with extensive data emerging from clinical trials, can we confidently claim ACEis and ARBs have the same beneficial downstream effect? This will be analyzed in extensive detail in a subsequent installment of this editorial.
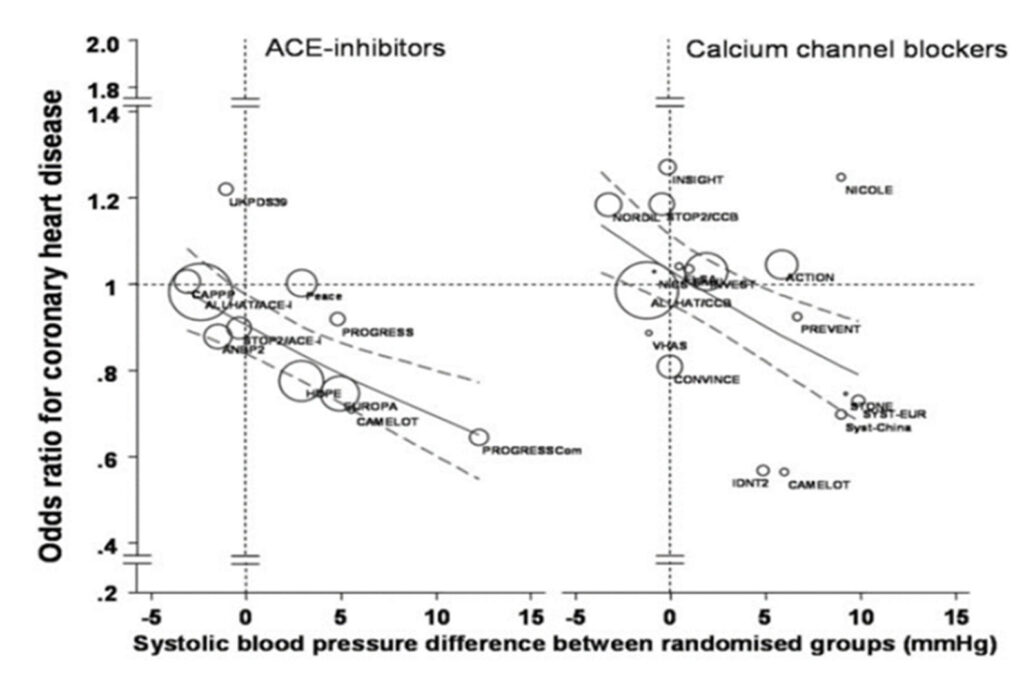
This matched cohort meta regression analysis was done based on extracted summary statistics regarding CHD and stroke from 28 outcome trials that compared either ACEIs or CCBs with diuretics, -blockers, or placebo for a total of 179122 patients, 9509 incident cases of CHD, and 5971 cases of stroke. CHD included myocardial infarction and coronary death.
REFERENCES
1. The Consensus Trial Study Group (1987) Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (Consensus). N Engl J Med
316:1429–1435. CrossRef Pubmed
2. The SOLVD Investigators (1991) Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 325:293–302. CrossRef Pubmed
3. Pfeffer M., Braunwald E., Moye L., et al. (1992) Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the Survival and Ventricular Enlargement Trial. The SAVE Investigators. N Engl J Med 327:669–677. CrossRef Pubmed
4. Cohn J., Tognoni G. and. Valsartan Heart Failure Trial Investigators (2001) A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 345:1667–1675. CrossRef Pubmed
5. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators (1993) Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. Lancet 342:821–828. Pubmed
6. Kober L., Torp-Pedersen C., Carlsen J., et al. (1995) A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med 333:1670–1676. CrossRef Pubmed
7. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group (1995) ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. Lancet 345:669–685. Pubmed
8. Yusuf S., Sleight P., Pogue J., et al. (2000) Effects of an angiotensin-converting- enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 342:145–153. CrossRef Pubmed
9. Fox K. and EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators (2003) Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled,
multicentre trial (the EUROPA study). Lancet 362:782–788. CrossRef Pubmed
10. Hansson L., Lindholm L., Niskanen L., et al. (1999) Effect of angiotensin-converting- enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: The Captopril Prevention Project (CAPPP) Randomised Trial. Lancet 353:611–616. CrossRef Pubmed
11. Doggrell S. (2003) ACE inhibitors versus diuretics: ALLHAT versus ANBP2. Expert Opin Pharmacother 4:825–828. CrossRef Pubmed
12. Beckett N., Peters R., Fletcher A., et al. (2008) Treatment of hypertension in patients 80 years of age or older. N Engl J Med 358:1887–1898. CrossRef Pubmed
13. Dahlof B., Sever P., Poulter N., et al. (2005) Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial – Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet
366:895–906. CrossRef Pubmed
14. Heart Outcomes Prevention Evaluation Study Investigators (2000) Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 355:253–259. Pubmed
15. Lewis E., Hunsicker L., Bain R., Rohde R. (1993) The effect of angiotensin-converting- enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329:1456–1462. CrossRef Pubmed
16. Chalmers J., Perkovic V., Joshi R., et al. (2006) Advance: breaking new ground in type 2 diabetes. J Hypertens Suppl 24:S22–S28. CrossRef Pubmed
17. Verdecchia P, Reboldi G, Angeli F, et al. Angiotensin-converting enzyme inhibitors and calcium channel blockers for coronary heart disease and stroke prevention. Hypertension. 2005;46:386–392. CrossRef Pubmed
18. Wright, Jr JT, Bakris G, et al. Effect of Blood Pressure Lowering and Antihypertensive Drug Class on Progression of Hypertensive Kidney Disease: Results From the AASK Trial. JAMA. 2002;288(19):2421–2431. CrossRef Pubmed
19. Cosentino F, Grant PJ, Aboyans V, et al; 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020 Jan 7;41(2):255-323. CrossRef Pubmed
20. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021 Apr 7;42(14):1289-1367. CrossRef Pubmed
21. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020 Jan 14;41(3):407-477. CrossRef Pubmed
22. Acute coronary syndromes. London: National Institute for Health and Care Excellence (UK);2020 Nov 18. Pubmed
23. McDonagh TA, Metra M, Adamo M, et al.; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599-3726. CrossRef Pubmed
Copyright Information
