Original Article
August 2023, 32:3
First online: 21 August 2023
Periodic Review Article
Pacing Induced Cardiomyopathy: Diagnosis and Management
Sidhi Laksono, 1, 2 Irwan Surya Angkasa, 3 Cliffian Hosanna 3
2 Department of Cardiology and Vascular Medicine, RS Siloam Jantung Diagram. Address: Jl. Cinere Raya No.19, Pangkalan Jati, Subdistrict Cinere, Depok city, West Java, Indonesia. Zip Code: 16514
3 Faculty of Medicine Tarumanagara University, Jl. Letjen S. Parman No.1, RT.3/RW.8, Tomang, Subdistrict Grogol petamburan, West Jakarta, Jakarta, Indonesia. Zip code:11440
Correspondence: Sidhi Laksono, Address: Fakultas Kedokteran Universitas Muhammadiyah Prof Dr HAMKA, Jl. Raden Patah No.01, RT.002/RW.006, Parung Serab, Subdistrict Ciledug, Tangerang City, Banten Province. Zip Code: 13460, Telephone: +628111585599,
Email: sidhilaksono@uhamka.ac.id,
Orcid ID: https://orcid.org/0000-0002-2959-8937
ABSTRACT
Permanent pacemaker is the mainstay treatment indicated for bradycardia caused by sinus node dysfunction. However, heart failure may appear in patients with chronic right ventricle pacing, this is known as Pacing-Induced Cardiomyopathy (PICM). There is no internationally accepted definition for diagnosis of PICM. Studies show the prevalence of PICM is 9% in the first year and increases in proportion to the duration of PPM implantation, but varies widely according to diagnostic criteria used. PICM causes a higher all-cause death, hospital admission, and cardiac death. Therefore, detecting risk factors may be an important part of the prevention and early treatment of PICM. Once PICM develops, several therapy options are available but Cardiac Resynchronization Therapy with biventricular Pacemaker is currently the forefront of treatment. But insight into other more novel therapeutic options such as; His bundle pacing and Left Bundle Branch Pacing shows promising results as an alternative treatment option in the near future.
Keywords
Ventricular dyssynchrony, heart failure, Pacing Induced
Cardiomyopathy, Cardiac Resynchronization Therapy
INTRODUCTION
Pacemaker implantation is indicated for bradycardia caused by sinus node dysfunction (SND) as chronic therapy when other potential treatable or reversible etiologies have been excluded. Pacemaker implantation is intended to increase heart rate and improve symptoms.1 However, long-term use of right ventricle pacing is reported to induce ventricular dyssynchrony which causes disturbances in left ventricular systolic function which further leads to heart failure syndrome or what is called pacing-induced cardiomyopathy (PICM).2 PICM is defined as a heart failure syndrome characterized by: (i). left ventricular systolic dysfunction (LVEF < 50%) and LVEF reduction ≥10% after pacemaker implantation or (ii). No other cause of left ventricular dysfunction. Incidence of PICM is estimated to occur in 5-20% of patients using right ventricular pacing for more than two years.3, 4 Symptoms that may appear in patients who develop PICM include dyspnea on exertion, paroxysmal nocturnal dyspnea, unexplained weight gain, and edema on the extremity. Physical examination may show signs of jugular venous pressure elevation, auscultatory crackles, S3 gallop, ascites, pulmonary edema, or pleural effusion.4 Patients with PICM have higher probability of all cause death, HF admission, and cardiac death compared to non-PICM, so observation and intervention are needed.5 This study was conducted to provide the latest updates on diagnosing patients with PICM and the best management that can be done in patients by looking at the conditions in each hospital.
Predictors and Risk Factors of Pacing Induced Cardiomyopathy (PICM)
Patient who undergoes RV pacing has up to 26% chance develop LV systolic dysfunction. Many studies have been conducted to find out about the risk factors for PICM. These studies found that men are more likely to experience PICM than women. History of heart diseases such as myocardial infarction, atrioventricular block, atrial fibrillation, and pre-existing LV systolic dysfunction.5–8 LBBB at baseline ECG, lower baseline LVEF, wider paced QRS duration and pre-LVESD, and higher burden of right ventricular pacing were predictors of PICM.6, 7, 9, 10 The study shows patient with baseline LVEF under 55% before pacing, wider paced QRS duration (>160msec), and pacing burden more than 33% has a higher risk for developing PICM.9
Mechanism of Pacing-Induced Cardiomyopathy (PICM)
Permanent pacing is indicated for patients with total AV block (TAVB) as well as bradycardia caused by SND. Symptomatic SND requiring GDMT or with bradycardia with significant comorbid, single chamber ventricular pacing is recommended unless there is reason to avoid the RV lead (IIa).1 Studies have found that in patients with higher ventricular pacing burden (> 40%), it is more common to have a decreased LVEF, which causes symptoms of heart failure. This is supported by predictive factors such as lower baseline LVEF and wider-paced QRS duration.10, 11 Pacing in the distal part of the conduction system disrupts physiological conduction. Conduction does not pass through the his-purkinje system, but rather through the ventricular myocardium which causes ventricular dyssynchrony. Ventricular dyssynchrony furthermore causes abnormal contraction of the ventricle which leads to LV remodeling. Ventricular dilatation, functional MR, reduced LVEF, myocardial fibrosis, and neuro-hormonal activation play a role in LV remodeling.12 A study conducted by the author previously found that there were neuro-hormonal changes in the remodeling process of LV. The authors found that there was a decrease in miR-155, and an increase in interleukin-6 (IL-6), soluble tumor necrosis factor 2 (sTNFR-2), matrix metalloproteinase-9 (MMP-9), N-cadherin (N-Cad), Occludens Zone-1 (ZO-1).13
Diagnosis of Pacing Induced Cardiomyopathy (PICM)
PICM is generally defined as a fall in Left Ventricle Ejection Fraction (LVEF) after implantation of a cardiac pacemaker. This results in symptoms of heart failure such as; dyspnea on exertion, paroxysmal nocturnal dyspnea, unexplained weight gain, edema on extremity, and physical examination may show elevation of jugular venous pressure, auscultatory crackles, S3 gallop, ascites, pulmonary edema or pleural effusion. PICM happens mostly in patients with high right ventricle pacing burden and may happen in months to years following implantation of the pacemaker. No consensus has been reached in determining the diagnostic criteria of PICM. Current literature uses varying criteria for diagnosing PICM. A systematic review and meta-analysis done by Somma et al, states that there are 15 unique diagnostic criteria found in the current literature.6 Based on a review by Mizner et al, these are the most often used diagnostic criteria for PICM.14
-
1. Decrease of LVEF below 50%, regardless of patients’ symptoms, or a reduction of LVEF by 10% or more.
-
2. Decrease of LVEF below 45%, or reduction of LVEF by 10%
or more after implantation of pacemaker device.
-
3. Decrease of LVEF below 40%, or an indication of upgrading
to cardiac resynchronization therapy (CRT).
-
4. Reduction of LVEF by 5% or more, with signs and symptoms
of heart failure, without other etiology of heart failure.
It is estimated that about 6-22% of patients with a permanent pacemaker will fulfill the criteria for PICM within 3-16 years. The wide prevalence of PICM is attributed to differences in diagnostic criteria used, variability of patients included in the studies, and different follow-up duration.14 One study by Kaye et al, found that in 118 patients with permanent pacemakers and follow-up echocardiography with mean duration of three and a half years, using three unique definitions of PICM, the prevalence of PICM is 5.9% – 39%. This study demonstrates that just based on the diagnostic criteria used, the resulting prevalence may differ exponentially.11
Yu et al observed that after one year, 9% of individuals with a right ventricular pacemaker will develop PICM.15 Khurshid et al, reported a prevalence of 19.5% after a median followup of 3.3 years.4 Zhang et al also reported a prevalence of 26% in patients with a median follow-up of 7.8 years.16 These three studies mentioned found that the shortest time a patient develops PICM is one month after the implantation of the pacemaker, and the longest time a patient is diagnosed with PICM is nine years following the implantation of pacemaker, suggesting PICM may occur even years after PPM.4, 15, 16 A systematic review conducted by the author previously, found that the incidence of PICM increases proportionally with the duration of PPM.17
Early Detection of Ventricular Dyssynchrony
PICM may occur as soon as one month after PPM implantation.4 Therefore, detecting pathophysiological changes and identifying risk factors is crucial in the prevention and treatment of PICM.
RV pacing, especially with apex location of the pacemaker, induces a slow myocyte-to-myocyte electrical signal transmission. This electrical signal propagates slowly, through the myocardium and bypasses the physiological conduction system causing disproportional RV and LV contraction. Initial depolarization happens near the pacing site followed by delayed depolarization of more remote segments. This nonphysiological cardiac contraction is known as ventricular dyssynchrony.14
There are two different types of ventricular dyssynchrony, interventricular (between right and left ventricle) and intraventricular (within one side of the ventricle). Interventricular dyssynchrony can be visualized as delayed aortopulmonary valve opening times and can be assessed with routine Doppler echocardiographic imaging. While an intraventricular dyssynchrony is a delay of mechanical activation between the various segments of the ventricle, and is harder to assess, requiring a Tissue Doppler imaging (TDI) or 2D speckletracking strain analysis and real-time 3D echocardiography.14
Interventricular dyssynchrony is identified to be an important predictor of ≥10% decrease in LVEF along with high burden RV pacing (>60%) in a study by Bansal et al.18 Another parameter in echocardiography that may predict LV dysfunction which precedes a decrease in LVEF is the Global Longitudinal Scale (GLS). GLS using 2D echocardiography has high sensitivity (92%) and specificity (89%) for detecting early LV systolic dysfunction. Iqbal et al demonstrate that there is a significant decrease in GLS of patients with PPM after one month, especially those with high RV pacing burden (40%) and apical pacemaker location.19 This is supported by the PAVD study which found one-month GLS reduction to be highly predictive in assessing patients after implantation of PPM with risk of LV dysfunction and subsequent cardiomyopathy.20
Traditionally, ventricular dyssynchrony is assessed with a simple 12-lead ECG. Wide-paced QRS duration (QRSd) (>160msec) is identified as a predictor for PICM. Unfortunately, QRSd is limited as to what it can provide as it is not able to assess separate right and left ventricle activation.14 Vectorcardiography measuring QRS Area (QRSa) is a parameter that may be acquired using a 12-lead ECG or orthogonal chest leads. Vectorcardiography too is unable to assess separate right and left ventricle activation but may help in CRT optimization as large QRS areas have been found to be associated with better CRT responses and failure to achieve large QRS area reduction is associated with poorer survival and echocardiographic outcomes.21, 22
Other more complex and non-invasive modalities to assess ventricular dyssynchrony are the ECG belt system (EBS) and ECG imaging (ECGi). Both use body surface potential mapping (BSPM) to measure a variety of parameters. EBS uses 53 body surface electrodes to produce isochronal activation maps reflecting spatial propagation of ventricular activation as reflected on the body surface.23 While an ECGi is a more complex and resource-consuming modality, needing 252 body surface electrodes and a CT-Scan to construct 1500 epicardial unipolar electrograms.24 Both are currently still novel technologies and see the bulk of their use in research rather than clinical settings.
In accordance with the results of the author’s previous study which found neurohormonal changes in patients after ppm with LV dysfunction assessed by GLS, the author suggests measuring these biomarkers to assess LV dysfunction in patients after PPM implantation.13
Management for Pacing Induced Cardiomyopathy (PICM)
There are many management that can be done in patients who experience PICM. Studies were conducted to determine the best choice for PICM patients who can provide the best benefit with the lowest risk. Patients with PICM who underwent an upgrade to a single or dual chamber biventricular pacing (cardiac resynchronization Therapy/CRT), His Bundle pacing (HBP), and Left bundle branch pacing (LBBP) showed improvements especially in LVEF, symptoms of heart failure, and quality of life although each action has its advantages and disadvantages.
Cardiac Resynchronization Therapy (CRT)
ESC guidelines recommend upgrading to CRTs in patients with conventional pacemakers or an ICD who develop refractory heart failure and LVEF ≤35% and for those with significant RV pacing burden. (Class IIa level B). CRT itself can prevent PICM from happening and more superior to RV pacing in relieving symptoms. Biventricular pacing is proven to be effective and safe in patients with LBBB, as it should remain as first-line therapy. On the other hand, HBP has shown better results in patients with RBBB.2
Biventricular pacing (BVP)
Biventricular pacing was first introduced in 1979 to assess arrhythmia. While in 1987, the concept of biventricular was granted a patent then to be widely used. Beginning in 1993, biventricular pacing already shows that this procedure improves functional capacity and LV function.25 As science develops and research is conducted on BVP, it is found that upgrading to BVP provides many benefits in treating patients with PICM. BVP shows improvement on LVEF, NYHA class, walking distance, and Quality of Life, furthermore, reduces the LV remodeling process.26–28 A systematic review reported a comparison of the use of BVP and RVP in patients with atrioventricular conduction defects. As a result, it was found that the use of BIV significantly reduced the mortality rate, as well as the incidence of hospitalization due to HF.26 Transient or permanent loss of biventricular pacing has been reported in some patients. This happens mostly due to Lead dislodgement so reinstallation is required. T-waves oversensing (TWOS) is also seen in some patients. Some old studies suggested a decrease in the ventricular sensitivity (from 0.3 to 0.45 mV or more) and after this was done, no further TWOS was seen.29, 30 However, reduced ventricular sensitivity can affect the ability of the device to detect VF. Recent studies have shown that reducing ventricular sensitivity is not necessary. This can be avoided by increasing the post-pacing blanking period. That way, the sensitivity does not need to be lowered so that the device can still detect VF.31
His Bundle Pacing (HBP)
Upgrading from right ventricular pacing to cardiac resynchronization Therapy in patients with PICM, HBP can be superior to BVP. HBP achieves physiological pacing by activating the ventricle via native conduction system. That way, PICM can be avoided.32 A study by Gardas R et al. shows how HBP is superior to BVP. The group of patients who underwent HBP showed an increase in LVEF from 34.3% to 48.2% after six months of follow-up compared to the group of patients who underwent BVP, which only reached 43.9% from the baseline of 32.9%. Moreover, the study shows improvement in NYHA class, LVEF, and mitral regurgitation more common in patients with HBP.33 Although all benefit that HBP offer, it has disadvantages such as technical difficulties, reduced R wave amplitudes, and high and unstable threshold. The success rate of HBP reported at ranges from 72 to 92% with re-intervention rate at 6 to 8%. Compared to LBBP which has more than 80% success rate with low re-intervention rate.32, 34, 35
Left Bundle Branch Pacing (LBBP)
Among various patient populations with low and stable thresholds, LBBP shows promising results, better in safety, efficacy, and outcomes rather than BVP or HBP.36–39 This technique is done by placing a pacing device 10-15 mm below his bundle region using an imaginary line drawn from the distal extent of the his bundle to the RV apex.40 This technique can be an option for patients who develop signs of PICM after long time use of right ventricular pacing. Furthermore, LBBP can produce a near-physiological or true conduction system while bypassing the pathological or vulnerable region in the cardiac conduction system.41 A cohort study conducted by Li H, et al used 10 patients with PICM that upgraded to LBBP. This study aims to find out how cardiac function and quality of life (QoL) change after upgrading to LBBP. As a result, one month after the procedure, the patient’s LVEDD and CTR were lower than before. LVEF was also found to be increased. The 6-minute walking test (6 MWT) was found to increase which means heart failure is relieved and cardiac function is significantly improved.42 A case reported by Yang D et al, about An 86-yearold Chinese woman with high-degree atrioventricular block who undergo LBBP after two years using a dual chamber pacemaker. The study shows that within 1 week, there is a significant improveme nt in LVEF after post-operation and the QRS complex was significantly narrowed from 152 ms to 105 ms. 6 MWT improved, and the Minnesota Heart Failure Quality of Life scale score decreased indicating that the patient’s QoL was significantly enhanced.43 Though rare, septal perforation and thromboembolism can occur. As the operator, it is important to evaluate the thickness of the basal interventricular septum and lead length. If perforation occurs, the lead need to be re-implanted at different site. When the lead is appropriately repositioned, it is not associated with major adverse event.40, 44
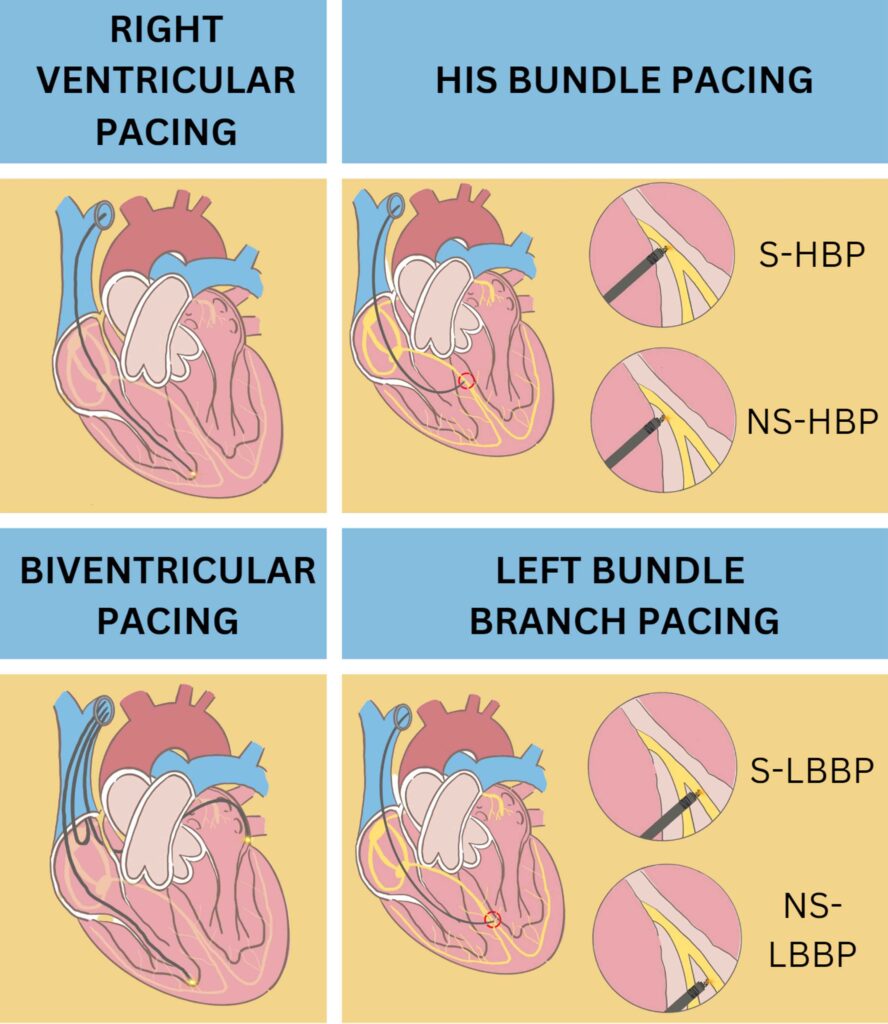
S-HBP= Selective His-Bundle Pacing; NS-HBP= Non-Selective His-Bundle Pacing; S-LBBP= Selective Left Bundle Branch Pacing; NS-LBBP= Non Selective Left Bundle Branch Pacing
CONCLUSION
PICM is defined as a heart failure syndrome characterized by: (i). left ventricular systolic dysfunction (LVEF < 50%) and LVEF reduction ≥10% after pacemaker implantation or (ii). No other cause of left ventricular dysfunction. LBBB at baseline ECG, lower baseline LVEF, wider paced QRS duration and pre-LVESD, and higher burden of right ventricular pacing were predictors of PICM. Echocardiography, ECG, and GLS examination can also be modalities for assessing a person’s risk of developing PICM. Furthermore, GLS can reveal dysfunction of the left ventricle even though there has not been a decrease in LVEF. Several biomarker changes were also found in patients who developed PICM. ESC guidelines recommend upgrading to CRTs in patients with a conventional pacemaker or an ICD who develop refractory heart failure and LVEF ≤35% and for those with significant RV pacing burden. BVP is the recommended pacing technique as the first choice, but at present, developments in the HBP and LBBP techniques show better results, both in terms of increasing LVEF, 6MWT, and quality of life. Compared to HBP, LBBP has more than 80% success rate with low re-intervention rate. Further studies and research are needed that can support LBBP to be the first choice in patients with AV block and SND who need CRT.
Conflict of Interest: None
Financial disclosure statement: None
REFERENCES
1. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MR, et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients With Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019 Aug
20;140(8):e382-482. CrossRef Pubmed
2. Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy [published correction appears in Eur Heart J. 2022 May 1;43(17):1651]. Eur Heart J. 2021;42(35):3427-3520. CrossRef Pubmed
3. Kiehl EL, Makki T, Kumar R, Gumber D, Kwon DH, Rickard JW, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016 Dec;13(12):2272-8. CrossRef Pubmed
4. Khurshid S, Epstein AE, Verdino RJ, Lin D, Goldberg LR, Marchlinski FE, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2014 Sep;11(9):1619-25. CrossRef Pubmed
5. Cho SW, Gwag H Bin, Hwang JK, Chun KJ, Park KM, On YK, et al. Clinical features, predictors, and long-term prognosis of pacing-induced cardiomyopathy. Eur J Heart Fail. 2019 May 1;21(5):643-51. CrossRef Pubmed
6. Somma V, Ha FJ, Palmer S, Mohamed U, Agarwal S. Pacing-induced cardiomyopathy: A systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm. 2023 Feb;20(2):282-90. CrossRef Pubmed
7. Zhang H, Zhou YJ, Zeng YJ. Prognostic factors of pacing-induced cardiomyopathy. Chin Med J (Engl). 2020;133(13):1533-9. CrossRef Pubmed
8. Naqvi TZ, Chao CJ. Adverse effects of right ventricular pacing on cardiac function: prevalence, prevention and treatment with physiologic pacing. Trends Cardiovasc Med. 2023;33(2):109-122. CrossRef Pubmed
9. Perla H, Chandra S, Patloori S, Chase D, Jacob J, Vellore C. Do the predictors of right ventricular pacing-induced cardiomyopathy add up? 2020; Available from: https://doi.org/10.22541/au.159969825.51344659 CrossRef Pubmed
10. Lee SA, Cha MJ, Cho Y, Oh IY, Choi EK, Oh S. Paced QRS duration and myocardial scar amount: predictors of long-term outcome of right ventricular apical pacing. Heart Vessels. 2016 Jul 1;31(7):1131–9. CrossRef Pubmed
11. Kaye G, Ng JY, Ahmed S, Valencia D, Harrop D, Ng ACT. The Prevalence of Pacing-Induced Cardiomyopathy (PICM) in Patients With Long Term Right Ventricular Pacing − Is it a Matter Of Definition? Heart Lung Circ. 2019 Jul 1;28(7):1027-33. CrossRef Pubmed
12. Abbas J, Zulqarnain M, Waqar F, et al. Incidence and predictors of pacemaker-induced cardiomyopathy with right ventricular pacing: a systematic review. Expert Rev Cardiovasc Ther. 2022;20(4):267-273. CrossRef Pubmed
13. Laksono S, Yuniadi Y, Soesanto AM, Harahap AR, Halomoan R, Kusharsamita H. Mechanism of Pacemaker-Induced Left Ventricular Dysfunction: Study Protocol. Journal of Hunan University Natural Sciences. 2022 Feb 28;49(2):180-9. CrossRef
14. Mizner J, Jurak P, Linkova H, Smisek R, Curila K. Ventricular Dyssynchrony and Pacing-induced Cardiomyopathy in Patients with Pacemakers, the Utility of Ultra-high-frequency ECG and Other Dyssynchrony Assessment Tools. Vol. 11, Arrhythmia and Electrophysiology Review. Radcliffe Medical Media; 2022. CrossRef Pubmed
15. Yu CM, Chan JYS, Zhang Q, Omar R, Yip GWK, Hussin A, et al. Biventricular Pacing in Patients with Bradycardia and Normal Ejection Fraction. New England Journal of Medicine. 2009 Nov 26;361(22):2123-34. CrossRef Pubmed
16. ZHANG XH, CHEN H, SIU CW, YIU KH, CHAN WS, LEE KL, et al. New-Onset Heart Failure After Permanent Right Ventricular Apical Pacing in Patients with Acquired High-Grade Atrioventricular Block and Normal Left Ventricular Function. J Cardiovasc Electrophysiol. 2008 Feb;19(2):136-41. CrossRef Pubmed
17. Laksono S, Setianto B, Iqbal M, Prawara AS. Understanding Pacemaker- Induced Cardiomyopathy Incidence and Predictors in Patients with Right Ventricular Pacing: A Systematic Review. International Journal of Angiology. 2022 Mar 1;31(01):010-5. CrossRef Pubmed
18. Bansal R, Parakh N, Gupta A, Juneja R, Naik N, Yadav R, et al. Incidence and predictors of pacemaker-induced cardiomyopathy with comparison between apical and non-apical right ventricular pacing sites. Journal of Interventional Cardiac Electrophysiology. 2019 Oct 1;56(1):63–70. CrossRef Pubmed
19. Iqbal M, Endamatriza GR, Lampita I, et al. The Influence of Right Ventricular Pacing Location, Pacing Burden and Paced QRS Duration to Subclinical Left Ventricular Systolic Dysfunction as Shown by Global Longitudinal Strain Echocardiography. Acta Med Indones. 2021;53(3):245-253. Pubmed
20. Ahmed FZ, Motwani M, Cunnington C, Kwok CS, Fullwood C, Oceandy D, et al. One-month global longitudinal strain identifies patients who will develop pacing-induced left ventricular dysfunction over time: The pacing and ventricular dysfunction (PAVD) Study. PLoS One. 2017 Jan 1;12(1). CrossRef Pubmed
21. Ghossein MA, van Stipdonk AMW, Plesinger F, Kloosterman M, Wouters PC, Salden OAE, et al. Reduction in the QRS area after cardiac resynchronization therapy is associated with survival and echocardiographic response. J Cardiovasc Electrophysiol. 2021 Mar 1;32(3):813-22. CrossRef Pubmed
22. van Stipdonk AMW, Ter Horst I, Kloosterman M, Engels EB, Rienstra M, Crijns HJGM, et al. QRS Area Is a Strong Determinant of Outcome in Cardiac Resynchronization Therapy. Circ Arrhythm Electrophysiol. 2018 Dec 1;11(12):e006497. CrossRef Pubmed
23. Gage RM, Curtin AE, Burns K V., Ghosh S, Gillberg JM, Bank AJ. Changes in electrical dyssynchrony by body surface mapping predict left ventricular remodeling in patients with cardiac resynchronization therapy. Heart Rhythm. 2017 Mar 1;14(3):392-9. CrossRef Pubmed
24. Ploux S, Lumens J, Whinnett Z, Montaudon M, Strom M, Ramanathan C, et al. Noninvasive electrocardiographic mapping to improve patient selection for cardiac resynchronization therapy: Beyond QRS duration and left bundle branch block morphology. J Am Coll Cardiol. 2013 Jun 18;61(24):2435-43. CrossRef Pubmed
25. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64(10):1047-1058. CrossRef Pubmed
26. Lu D, Zhang H, Chen C, Wang K, Shan Q. Clinical outcomes with biventricular versus right ventricular pacing in patients with atrioventricular conduction defects. Heart Failure Reviews. 2018 Nov;23(6):897-906. CrossRef Pubmed
27. Beck H, Curtis AB. Right Ventricular Versus Biventricular Pacing for Heart Failure and Atrioventricular Block. Vol. 13, Current Heart Failure Reports. Current Science Inc.; 2016. p. 230-6. CrossRef Pubmed
28. Ruwald AC, Kutyifa V, Ruwald MH, Solomon S, Daubert JP, Jons C, et al. The association between biventricular pacing and cardiac resynchronization therapy-defibrillator efficacy when compared with implantable cardioverter defibrillator on outcomes and reverse remodelling. Eur Heart J. 2015 Feb 14;36(7):440-8. CrossRef Pubmed
29. Colchero T, Arias MA, López-Sánchez FA, Pachón M, Domínguez-Pérez L, Puchol A, et al. Loss of Continuous Biventricular Pacing in Cardiac Resynchronization Therapy Patients: Incidence, Causes, and Outcomes. Revista Española de Cardiología (English Edition). 2013 May;66(5):377-83. CrossRef Pubmed
30. Korantzopoulos P, Kyrlas K, Goudevenos JA. Loss of biventricular pacing due to T wave oversensing: Case report and review of the literature. Int J Cardiol. 2013 Sep;168(2):1626-8. CrossRef Pubmed
31. Kabunga P, Klein GJ, Hodkinson E, Sandgren C, Sy RW. Loss of Biventricular Pacing during Exercise: What Is the Mechanism? J Cardiovasc Electrophysiol. 2016 Mar 1;27(3):362-5. CrossRef Pubmed
32. Archontakis S, Sideris K, Laina A, et al. His bundle pacing: A promising alternative strategy for anti-bradycardic pacing – report of a singlecenter experience. Hellenic J Cardiol. 2022;64:77-86. CrossRef Pubmed
33. Gardas R, Golba KS, Soral T, Biernat J, Kulesza P, Sajdok M, et al. The Effects of His Bundle Pacing Compared to Classic Resynchronization Therapy in Patients with Pacing-Induced Cardiomyopathy. J Clin Med. 2022 Oct 1;11(19). CrossRef Pubmed
34. Yuan Z, Cheng L, Wu Y. Meta-Analysis Comparing Safety and Efficacy of Left Bundle Branch Area Pacing Versus His Bundle Pacing. Am J Cardiol. 2022 Feb;164:64-72. CrossRef Pubmed
35. Arnold AD, Whinnett ZI, Vijayaraman P. His-Purkinje conduction system pacing: State of the art in 2020. Arrhythm Electrophysiol Rev. 2020;9(3). CrossRef Pubmed
36. Ye Y, Wu S, Su L, Sheng X, Zhang J, Wang B, et al. Feasibility and Outcomes of Upgrading to Left Bundle Branch Pacing in Patients With Pacing-Induced Cardiomyopathy and Infranodal Atrioventricular Block. Front Cardiovasc Med. 2021 Jun 14;8. CrossRef Pubmed
37. Wang Y, Zhu H, Hou X, Wang Z, Zou F, Qian Z, et al. Randomized Trial of Left Bundle Branch vs Biventricular Pacing for Cardiac Resynchronization Therapy. J Am Coll Cardiol. 2022 Sep 27;80(13):1205-16. CrossRef Pubmed
38. Chen X, Ye Y, Wang Z, Jin Q, Qiu Z, Wang J, et al. Cardiac resynchronization therapy via left bundle branch pacing vs. optimized biventricular pacing with adaptive algorithm in heart failure with left bundle branch block: a prospective, multi-centre, observational study.
Europace. 2022 May 1;24(5):807-16. CrossRef Pubmed
39. Hua J, Wang C, Kong Q, Zhang Y, Wang Q, Xiong Z, et al. Comparative effects of left bundle branch area pacing, His bundle pacing, biventricular pacing in patients requiring cardiac resynchronization therapy: A network meta-analysis. Clin Cardiol. 2022 Feb 1;45(2):214-23. CrossRef Pubmed
40. Liu P, Wang Q, Sun H, Qin X, Zheng Q. Left Bundle Branch Pacing: Current Knowledge and Future Prospects. Front Cardiovasc Med. 2021;8:630399. Published 2021 Mar 23. CrossRef Pubmed
41. Zhang S, Zhou X, Gold MR. Left Bundle Branch Pacing: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74(24):3039-3049. CrossRef Pubmed
42. Li H, Wang L, Peng X, Wu J. The quality of life of patients with pacemaker-induced cardiomyopathy after they upgrade to left bundle branch pacing. Am J Transl Res. 2021;13(4):3044-3053. Pubmed
43. Yang D, Zhu H, Ma M, Wang X, Pan X. A case of pacing-induced cardiomyopathy dramatically reversed by left bundle branch pacing in one week. HeartRhythm Case Rep. 2021 Nov 1;7(11):762-6. CrossRef Pubmed
44. Raymond-Paquin A, Padala SK, Ellenbogen KA. Left Bundle Branch
Pacing: A Perfect Compromise? Arrhythm Electrophysiol Rev. 2021;10(4):241-3. CrossRef Pubmed
Copyright Information
